Abstract
A new Schiff base ester, 4-{[(4-iodophenyl)imino]methyl}phenyl octadecanoate was synthesized and its IR, 1H NMR, 13C NMR and EI-MS spectroscopic data are presented.
Liquid crystalline materials have many practical applications in scientific and technological areas, in particular as display devices, organic light emitting diodes, anisotropic networks, photoconductors and semiconductor materials [1,2,3]. High demand of new liquid crystals for applications has led to the preparation and study of numerous mesogens in particular, thermotropic liquid crystals [4,5]. In our previous studies, the results revealed that azomethine and ester groups are useful connecting units for generating mesomorphism in thermotropic liquid crystals with two and three aromatic rings. Aromatic azomethine ester comprising of different polarity of substituents has been known to either promote or suppress the mesomorphic properties [6,7,8]. As a continuation of our previous work, Schiff base esters and iodo terminal moieties are incorporated to form a new compound, 4-{[(4-iodophenyl)imino]methyl}phenyl octadecanoate.
Experimental
Analytical data were obtained on Perkin Elmer 2400 LS series CHNS/O analyzers. Electron impact mass spectra (EI-MS) were recorded by Hewlett Packard 5989A Mass Spectrometer operating at 70 eV ionizing energy. FT-IR data were recorded on a Perkin Elmer 2000-FTIR spectrophotometer. NMR spectra were recorded in CDCl3 on a Bruker 400 MHz Ultrashield Spectrometer.
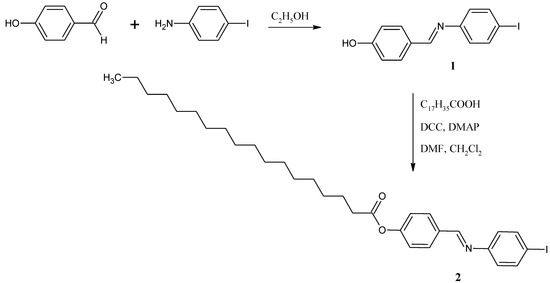
Scheme 1.
Synthesis of 4-{[(4-iodophenyl)imino]methyl}phenyl octadecanoate.
In analogy to a recently published procedure [9,10,11,12], a solution of 4-hydroxybenzaldehyde (1.22 g, 10 mmol) and 4-iodoaniline (2.19 g, 10 mmol) in absolute ethanol (70 mL) was heated under reflux for 3 h. Schiff base 1 thus obtained was recrystallized from absolute ethanol. Then, Schiff base 1 (0.66 g, 2 mmol) in dimethylformamide (DMF) (2 mL), was added into a solution of octadecanoic acid (0.57 g, 2 mmol) and 4-dimethylaminopyridine (DMAP) (0.12 g, 1 mmol) in dichloromethane (20 mL). The resulting mixture was stirred in an ice bath. To this solution, N,N’-dicyclohexylcarbodiimide (DCC) (0.41 g, 2 mmol) dissolved in dichloromethane (5 mL) was added dropwise while stirring in the ice bath for 1 h. The resulting mixture was subsequently stirred at room temperature for another 3 h. Then, the reaction mixture was filtered and the excess solvent was removed from the filtrate by evaporation. Recrystallization from absolute ethanol gave the Schiff base 2 as pale yellow solid (0.814 g, 69%).
Melting point: 109–110 °C.
MS (EI): m/z (rel. int. %) = 589 (8) (M+), 323 (100).
IR (KBr): νmax (cm−1), 2954, 2915, 2849 (C-H aliphatic), 1753 (C=O), 1617 (C=N), 1215, 1101 (C-O).
1H NMR (400 MHz, CDCl3): δ/ppm 0.90 (t, 3H, J = 6.6Hz, CH3-), 1.28 (m, 16H, CH3-(CH2)14-), 1.78 (qt, 2H, J = 7.4 Hz, -CH2-CH2-COO-), 2.60 (t, 2H, J = 7.5 Hz, -CH2-COO-), 6.98 (d, 2H, J = 8.7 Hz, Ar-H), 7.22 (d, J = 8.4 Hz, 2H, Ar-H), 7.72 (d, J = 8.7 Hz, 2H, Ar-H), 7.93 (d, J = 8.4 Hz, 2H, Ar-H), 8.67 (s, 1H, -CH=N-).
13C NMR (100 MHz, CDCl3,): δ/ppm 14.10 (CH3-), 22.68 (CH3CH2-), 24.88 (CH3CH2CH2-), 29.10, 29.24, 29.35, 29.45, 29.59, 29.65, 29.69 for methylene carbons (CH3CH2CH2-(CH2)12-), 31.92 (-CH2CH2COO-), 34.43 (-CH2COO-), 90.32, 122.10, 122.93, 130.07, 133.51, 138.19, 151.57, 153.37 for aromatic carbons, 159.50 (-CH=N-), 171.87 (-COO-).
Elemental analysis: Calculated for C31H44INO2, 63.15%, H, 7.52%, N, 2.38%; Found: C, 63.21%, H, 7.42%, N, 2.31%.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
Authors would like to thank Universiti Tunku Abdul Rahman and Universiti Sains Malaysia for the financial supports via UTAR Research Fund and research facilities.
References
- Petti, L.; Rippa, M.; Fiore, A.; Manna, L.; Mormile, P. Optically induced light modulation in an hybrid nanocomposite system of inorganic CdSe/CdS nanorods and nematic liquid crystals. Opt. Mater. 2010, 32, 1011–1016. [Google Scholar] [CrossRef]
- Shurpo, N.A.; Vakshtein, M.S.; Kamanina, N.V. Effect of CdSe/ZnS semiconductor quantum dots on the dynamic properties of nematic liquid-crystalline medium. Tech. Phys. Lett. 2010, 36, 319–321. [Google Scholar] [CrossRef]
- Hoang, M.H.; Cho, M.J.; Kim, K.H.; Lee, T.W.; Jin, J.I.; Choi, D.H. Semiconducting 2,3,6,7,10,11-hexakis{4-(5-dodecylthiophen-2-yl)phenyl]ethynyl}triphenylene and its discotic liquid crystalline properties. Chem. Lett. 2010, 39, 396–397. [Google Scholar] [CrossRef]
- Yuksel, F.; Atilla, D.; Ahsen, V. Synthesis and characterization of liquid crystalline unsymmetrically substituted phthalocyanines. Polyhedron 2007, 26, 4551–4556. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Meng, F.B.; Tian, M.; Xiao, W.Q. Side-chain liquid-crystalline polysiloxanes containing ionic mesogens and cholesterol ester groups. React. Funct. Polym. 2005, 66, 551–558. [Google Scholar] [CrossRef]
- Yeap, G.Y.; Ha, S.T.; Lim, P.L.; Boey, P.L.; Ito, M.M.; Sanehisa, S.; Youhei, Y. Synthesis, physical and mesomorphic properties of Schiff’s base esters containing ortho-, meta- and para-substituents in benzylidene-4’-alkanoyloxyanilines. Liq. Cryst. 2006, 33, 205–211. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Ong, S.T.; Yeap, G.Y.; Wong, J.P.W.; Koh, T.M.; Lin, H.C. Synthesis and mesomorphic properties of new Schiff base esters with different alkyl chains. Chin. Chem. Lett. 2009, 20, 767–770. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Sivasothy, Y.; Yeap, G.Y.; Boey, P.L.; Lin, H.C. New mesogenic Schiff base esters with polar chloro substituent: synthesis, thermotropic properties and x-ray diffraction studies. Am. J. Appl. Sci. 2010, 7, 214–220. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Synthesis of New Schiff Base: 4-[(Pyridin-3-ylmethylene)amino]phenyldodecanoate. Molbank 2008, 2008, M582. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. 4-[(Pyridin-3-ylmethylene)amino]phenylhexadecanoate. Molbank 2009, 2009, M584. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. 4-[(Pyridin-3-ylmethylene)amino]phenyltetradecanoate. Molbank 2009, 2009, M585. [Google Scholar] [CrossRef]
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. 4-[(Pyridin-3-ylmethylene)amino]phenyloctadecanoate. Molbank 2009, 2009, M591. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).