Abstract
Methylparabene (2) was simply benzamidomethylated with (benzamidomethyl)-triethylammonium chloride (1) in aqueous medium to afford methyl 4-(benzamido-methoxy)benzoate (3) in high yield. The title compound was characterized by elemental analysis, FT-IR, 1H-NMR and 13C-NMR spectroscopy.
This paper aims to present a compound similar to methyl 4-methoxybenzoate in which methyl from the methoxy group is replaced with a benzamidomethyl group. Methyl 4-methoxybenzoate, also known as methyl anisate, has been frequently used as pharmaceutical intermediate and in many organic syntheses [1] and in food as flavoring agent [2,3]. In addition, it can be found as volatile component in many plants and mushrooms [4,5,6,7,8].
Although (benzamidomethyl)triethylammonium chloride (1) is an excellent reagent for benzamido-methylation of phenols [9], in our previous work [10] we demonstrated that the phenol group at 4-hydroxybenzoic acid cannot be benzamidomethylated with 1 in aqueous media. The carboxylic group as a weak nucleophile in aqueous media does not react [11], but it deactivates the phenol group in the molecule of 4-hydroxybenzoic acid. Since in methylparabene (2) the carboxylic group is protected, the hydroxy group can be easily benzamidomethylated with 1 in aqueous media to give methyl 4-[(benzoylamino)methoxy]benzoate (3) (Scheme 1).
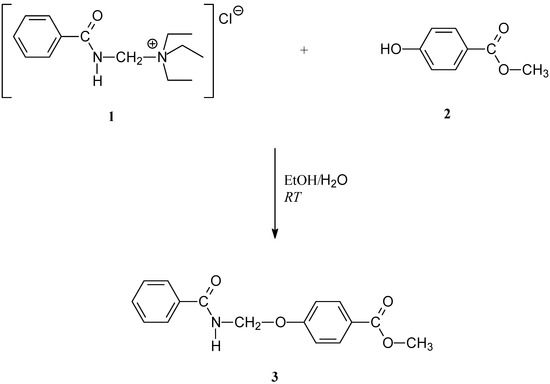
Scheme 1.
Synthetic route to the title compound 3.
Experimental
Compound 1 is not commercially available and it was synthesised as described previously [9].
Methyl 4-[(benzoylamino)methoxy]benzoate (3)
To a mixture of powdered 1 (1.058 g, 3.9 mmol), 2 (0.501 g, 3.3 mmol), ethanol (20 mL) and triethylamine (0.2 mL), water was continually added, drop by drop, until a clear solution was obtained. The mixture was stirred for 5 h at room temperature. Then, water was added to the mixture until occurrence of a precipitate. The typical yield of crude colorless crystals with mp of 133–140 °C was 80%. Purification was performed by dissolving the product in acetone followed by precipitation with water and subsequent recrystallization from toluene.
Melting point of pure crystals: 142–143 °C (uncorrected).
FT-IR (KBr): 3,290 (νNH), 1,718 (νOC=O), 1,663 (Amide I), 1,555 cm−1 (Amide II)
1H-NMR (250 MHz, DMSO-d6): δ/ppm 9.64 (t, J = 6.6 Hz, 1H, NH); 7.93–7.15 (9H, Ar); 5.41 (d, J = 6.6 Hz, 2H, N-CH2-O); 3.81 (s, 3H, CH3)
13C-NMR (63 MHz, DMSO-d6): δ/ppm 167.0 (C=O); 165.9 (C=O); 68.7 (CH2); 51.9 (CH3); Ar: 160.8, 133.3, 132.1, 131.2, 128.6, 127.5, 122.3, 115.2.
Anal. Calcd. (found) for C16H15NO4: C, 67.36 (67.55); H, 5.30 (5.41); N, 4.91 (5.07).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Wang, H.; Pullambhatla, M.; Guilarte, T.R.; Mease, R.C.; Pomper, M.G. Synthesis of [125I]iodoDPA-713: A new probe for imaging inflammation. Biochem. Biophys. Res Commun. 2009, 389, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Perriot, R.; Breme, K.; Meierhenrich, U.J.; Carenini, E.; Ferrando, G.; Baldovini, N. Chemical composition of French mimosa absolute oil. J. Agric. Food Chem. 2010, 58, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Panda, H. Perfumes and Flavours Technology Handbook; Asia Pacific Business Press Inc: Delhi, India, 2010. [Google Scholar]
- Rapior, S.; Breheret, S.; Talou, T.; Pélissier, Y.; Bessière, J.-M. The anise-like odor of Clitocybe odora, Lentinellus cochleatus and Agaricus essettei. Mycologia 2002, 94, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.G.; Flath, R.A. Volatile components of pineapple guava. J. Agric. Food Chem. 1989, 37, 734–736. [Google Scholar] [CrossRef]
- Hardy, P.J.; Michael, B.J. Volatile components of feijoa fruits. Phytochemistry 1970, 9, 1355–1357. [Google Scholar] [CrossRef]
- Shaw, J.G.; Ellingham, P.J.; Birch, E.J. Volatile constituents of feijoa-headspace analysis of intact fruit. J. Sci. Food Agric. 1983, 34, 743–747. [Google Scholar] [CrossRef]
- Ceballos, L.; Pino, J.A.; Quijano-Celis, C.E.; Dago, A. Optimization of a HS-SPME/GC-MS Method for determination of volatile compounds in some Cuban unifloral honeys. J. Food Quality 2010, 33, 507–528. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple method for benzamidomethylation of phenols in water solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-hydroxybenzoate. Molbank 2010, 2010, M658. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)-triethylammonium chloride 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).