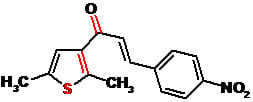
(2E)-1-(2,5-Dimethyl-3-thienyl)-3-(4-nitrophenyl)propenone
Abstract
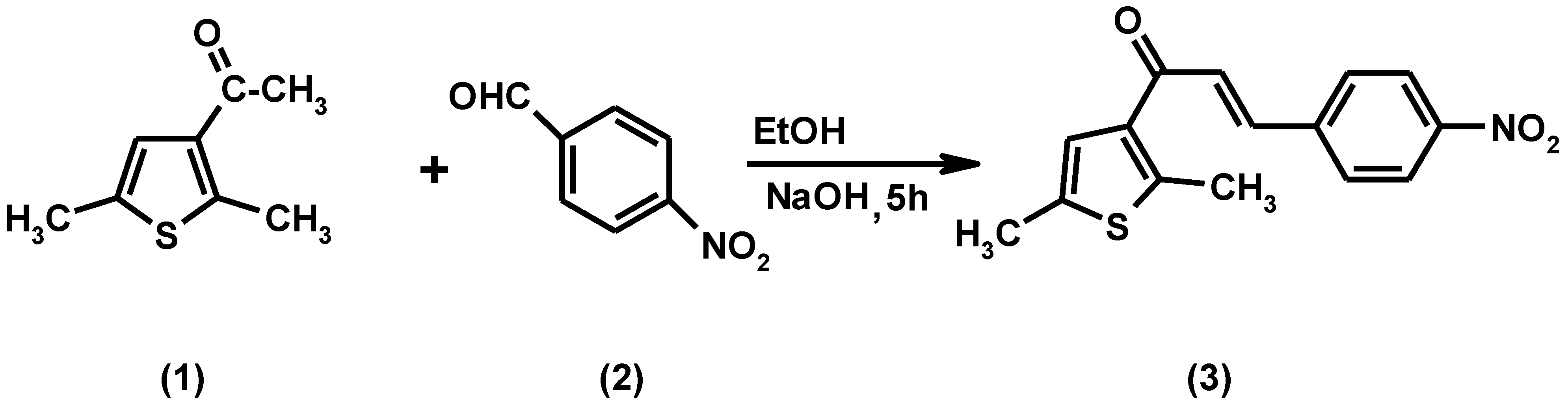
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Asiri, A.M.; Khan, A.S. 1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethyphenyl)prop-2-en-1-one. Molbank 2010, 2010, M692. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial pharmacodynamics of chalcone derivatives in combination with artemisinin against Plasmodium falciparum in vitro. Eur. J. Med. Chem. 2009, 44, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, C.G.; Ribas, J.C.; Devia, C.; Rodriguez, A.M.; Enriz, R.D. In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Kamal, A.; Ramakrishna, G.; Raju, P.; Viswanath, A.; Ramaiah, M.J.; Balakishan, G.; Pal-Bhadra, M. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorg. Med. Chem. Lett. 2010, 20, 4865–4869. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.N.; Wang, L.P.; Tao, R.X. Expression patterns of defence genes and antioxidant defence responses in a rice variety that is resistant to leaf blast but susceptible to neck blast. Physiol. Mol. Plant P. 2009, 74, 167–174. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Walter, C.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Selvakumar, N.; Kumar, G.S.; Azhagan, A.M.; Rajulu, G.G.; Sharma, S.; Kumar, M.K.; Das, J.; Iqbal, J.; Trehan, S. Synthesis, SAR and antibacterial studies on novel chalcone oxazolidinone hybrids. Eur. J. Med. Chem. 2007, 42, 538–583. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Steingrover, K.; Rao, M.S. Isolation and synthesis of chalcones with different degrees of saturation. Phytochemisy 2002, 61, 931–936. [Google Scholar] [CrossRef]
- Sharma, M.; Chaturvedi, V.; Manju, Y.K.; Bhatnagar, S.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Substituted quinolinyl chalcones and quinolinyl pyrimidines as a new class of anti-infective agents. Eur. J. Med. Chem. 2009, 44, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Batterjee, S.; Taib, L.A. Stereoselective crossed-aldol condensation of 3-acetyl-2,5-dimethylthiophene/furan with aromatic aldehydes in water: Synthesis of (2E)-3-aryl-1-(thien-3-yl/fur-3-yl)-prop-2-en-1-ones. Ind. J. Chem. B 2006, 45B, 1936–1941. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A.; Khan, K.A. (2E)-1-(2,5-Dimethyl-3-thienyl)-3-(4-nitrophenyl)propenone. Molbank 2011, 2011, M713. https://doi.org/10.3390/M713
Asiri AM, Khan SA, Khan KA. (2E)-1-(2,5-Dimethyl-3-thienyl)-3-(4-nitrophenyl)propenone. Molbank. 2011; 2011(1):M713. https://doi.org/10.3390/M713
Chicago/Turabian StyleAsiri, Abdullah M., Salman A. Khan, and Khalid A. Khan. 2011. "(2E)-1-(2,5-Dimethyl-3-thienyl)-3-(4-nitrophenyl)propenone" Molbank 2011, no. 1: M713. https://doi.org/10.3390/M713
APA StyleAsiri, A. M., Khan, S. A., & Khan, K. A. (2011). (2E)-1-(2,5-Dimethyl-3-thienyl)-3-(4-nitrophenyl)propenone. Molbank, 2011(1), M713. https://doi.org/10.3390/M713