Abstract
The title compound 2-[(3,5-dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]¬indane-1,3-dione (3) was synthesized in high yield by reaction of 3,5-dimethyl-1-phenyl¬pyrazole-4-carbaldehyde and indane-1,3-dione in ethanol in the presence of pyridine. The structure of this new compound was confirmed by elemental analysis, IR, 1H NMR, 13C NMR and GC-MS spectral analysis.
Naturally occurring as well as synthetic pyrazole-containing heterocyclic compounds have great importance for their biological activities such as anti-bacterial [1], anti-inflammatory [2], anti-hypertensive [3], anti-cancer [4], and anti-amoebic activity [5]. Pyrazole-containing donor-acceptor chromophores are also applicable in materials fields for their properties such as non-linear optical (NLO), optical limiting [6], electrochemical sensing [7] and langmuir film [8]. Due to the wide application of pyrazoles we decided to synthesize a new pyrazole-containing donor-acceptor chromophore by Knoevenagel condensation in analogy to a previously published procedure [9].
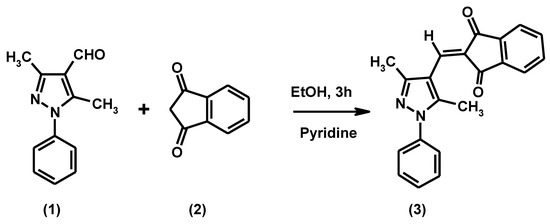
Scheme 1.
Synthesis of compound 3.
Experimental
Materials
3,5-Dimethyl-1-phenylpyrazole-4-carbaldehyde and indane-1,3-dione were purchased from Aldrich Chemicals. Melting points of the synthesized compounds were determined in open-glass capillaries on a Stuart-SMP10 melting point apparatus and are uncorrected. IR absorption spectra were recorded in the 4,000–400 cm−1 range on a Shimadzu FTIR-8400s using KBr pellets, 1H NMR and 13C NMR spectra were recorded on a Bruker-AVANCE-III 600 spectrometer at 600 MHz and 150 MHz, respectively, chemical shifts are reported as parts per million (ppm) downfield from TMS (Me4Si) used as an internal standard.
A mixture of 3,5-dimethyl-1-phenylpyrazole-4-carbaldehyde (1) (1.0 g, 0.005 mol), indane-1,3-dione (2) (0.73 g, 0.005 mol) and a few drops of pyridine in ethanol (15 mL) was heated for 3 h. The progress of the reaction was monitored by TLC. The solid that separated from the cooled mixture was collected and recrystallized from a methanol-chloroform mixture to give the title compound (3) as a yellow solid.
Yield: 85%; m.p. 196–197 °C
GC-MS m/z (rel. int. %): 330 (72) [M+1]+
IR (KBr) vmax cm−1: 3035 (Ar-H), 2859 (C-H), 1663 (C=O), 1578 (C=C)
1H NMR (DMSO-d6) (δ/ppm): 8.02 (d, J = 2.8 Hz), 7.97 (d, J = 2.8 Hz), 7.93 (s, C=CH), 7.82 (dd, J = 2.8, 2.8 Hz), 7.54 (m, 5H, Ar-H), 7.46 (dd, J = 6.6 Hz) 2.44 (s, CH3), 2.36 (s, CH3)
13CNMR (CDCl3) δ: 190.33, 188.65, 152.11, 144.83, 142.56, 140.10, 138.84, 136.16, 135.02, 134.76, 129.26, 128.11, 126.71, 125.07, 123.11, 122.86, 123.11, 122.86, 116.14, 14.14, 13.04.
Anal. calc. for C21H16N2O2: C, 76.81, H, 4.91, N, 8.53. Found: C, 76.79, H, 4.88, N, 8.84.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the Chemistry Department, King Abdul Aziz University, Jeddah, Saudi Arabia for providing the research facilities.
References
- Mitchell, R.E.; Greenwood, D.R.; Sarojini, V. An antibacterial pyrazole derivative from Burkholderia glumae, a bacterial pathogen of rice. Phytochemistry 2008, 69, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Loizzo, M.R.; Statti, G.A.; Michel, S.; Tillequin, F.; Menichini, F. The synthesis and Angiotensin Converting Enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Bonacorso, H.G.; Navarini, J.; Wiethan, C.W.; Bortolotto, G.P.; Paim, G.R.; Cavinatto, S.; Martins, M.A.P.; Zanatta, N.; Caro, M.S.B. The first application of 4-alkoxy-1,1,1-trifluoroalk-3-en-2-ones in a three-component condensation protocol for the synthesis of 3-acyl-4-aryl-2 (trifluoromethyl)-2-hydroxy-3,4,7,8-tetrahydro-2H-chromen-5(6H)-ones. J. Fluorine Chem. 2011, in press. [Google Scholar] [CrossRef]
- Sadr, M.H.; Sardroodi, J.J.; Zare, D.; Brooks, N.R.; Clegg, W.; Song, Y. Nonlinear optical properties and crystal structure determination of a pentanuclear copper(I) cluster (NEt4)2[MoS4(CuBp)4] (Bp = H2B(pyrazolyl)2). Polyhedron 2006, 25, 3285–3288. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Cui, Y.-P. The synthesis, structure and spectroscopic properties of novel oxazolone-, pyrazolone- and pyrazoline-containing heterocycle chromophores. Dye. Pigment. 2009, 81, 27–34. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, K.; Gong, F.; Li, S.; Chen, J.; Ma, J.S.; Sobenina, L.N.; Mikhaleva, A.I.; Trofimov, B.A.; Yang, G. A highly selective fluorescent sensor for fluoride anion based on pyrazole derivative: Naked eye “no–yes” detection. J. Photochem. Photobiol. A Chem. 2011, 217, 29–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. 2,6-Bis(9-ethyl-9H-carbazolylmethylene)cyclohexanone. Molbank 2009, 2009, M635. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).