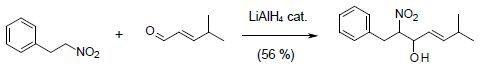6-Methyl-2-nitro-1-phenyl-hept-4-en-3-ol
Abstract
:Experimental
General
Synthesis of 6-methyl-2-nitro-1-phenyl-hept-4-en-3-ol (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Luzzio, F.A. The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Rosini, G.; Ballini, R. Functionalized Nitroalkanes as Useful Reagents for Alkyl Anion Synthons. Synthesis 1988, 1988, 833–847. [Google Scholar] [CrossRef]
- Youn, S.W.; Kim, Y.H. Facile Synthesis of 2-Nitroalkanols Mediated with LiAlH4 as Catalyst. Synlett 2000, 2000, 880–882. [Google Scholar]
- Kamitori, Y.; Hojo, M.; Masuda, R.; Inoue, T.; Izumi, T. Lithium Aluminum Hydride on Silica Gel - Selective Reduction of Ketones and Carboxy Esters in the Presence of Nitro and Cyano Groups. Tetrahedron Lett. 1983, 24, 2575–2576. [Google Scholar] [CrossRef]
- Gilbert, K.E.; Borden, W.T. Peracid Oxidation of Aliphatic Amines: General Synthesis of Nitroalkanes. J. Org. Chem. 1979, 44, 659–661. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Herrmann, J.M.; König, B. 6-Methyl-2-nitro-1-phenyl-hept-4-en-3-ol. Molbank 2011, 2011, M718. https://doi.org/10.3390/M718
Herrmann JM, König B. 6-Methyl-2-nitro-1-phenyl-hept-4-en-3-ol. Molbank. 2011; 2011(1):M718. https://doi.org/10.3390/M718
Chicago/Turabian StyleHerrmann, Josef M., and Burkhard König. 2011. "6-Methyl-2-nitro-1-phenyl-hept-4-en-3-ol" Molbank 2011, no. 1: M718. https://doi.org/10.3390/M718
APA StyleHerrmann, J. M., & König, B. (2011). 6-Methyl-2-nitro-1-phenyl-hept-4-en-3-ol. Molbank, 2011(1), M718. https://doi.org/10.3390/M718