Abstract
A new fluorescent di-imine compound containing two naphthalene groups has been synthesized by classical Schiff-base reaction between 2,2’-[1,3-phenylene-bis-(methyleneoxy)]dibenzaldehyde and 1-naphthylmethylamine. The new bischromophoric compound has been characterised by IR, NMR and MALDI-TOF MS spectroscopy. The photophysical characterization was carried out by UV-vis and fluorescence emission spectroscopy, using chloroform. The monomer and excimer bands, typical for the naphthalene in solution, are present in the emission spectra for the compound.
Introduction
The synthesis and characterisation of new fluorescence chemosensors has evolved rapidly in the last few years [1,2,3,4,5]. A fluorescence chemosensor is a compound that exhibits changes in its fluorescence properties upon interaction with an analyte. Fluorescence chemosensors have the potential to be highly selective even at very low concentrations of analyte [6]. The continuing research into the application of fluorescence probes has led to the development of numerous fluorescence systems based on restricting the rotation of imine (C=N) bonds by complexation of cations [7].
This paper describes the synthesis and characterisation of a new di-imine compound (L), which could potentially be used as fluorescence chemosensor due to its fluorescent properties. The compound has been prepared by classical Schiff-base reaction between 2,2’-[1,3-phenylenebis-(methyleneoxy)]-dibenzaldehyde (1) and the fluorescence probe 1-naphthylmethylamine (2). The synthetic route to prepare L is depicted in Figure 1.
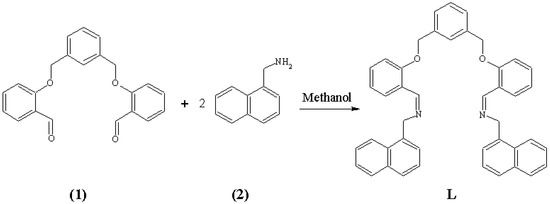
Figure 1.
Schematic synthesis of L.
Experimental
2,2’-[1,3-phenylenebis(methyleneoxy)]dibenzaldehyde (1) was prepared following the method described previously [8], by reaction between salicylaldehyde, sodium hydroxide and 1,3-bis(bromo-methyl)benzene (2:2:1 ratio) in an ethanol/water solution (2:1 ratio). A solution of 2,2’-[1,3-phenylene-bis(methyleneoxy)]dibenzaldehyde (1) (0.346 g), dissolved in methanol (50 mL) was added dropwise to a solution of 1-naphthylmethylamine (2) (0.157 mL) in methanol (50 mL). The resulting solution was gently refluxed with magnetic stirring for 4 h, during which the colour changed slowly from clear to pale yellow. The solution was then left to cool, whilst stirring, for 24 h. The solvent was then evaporated to ca. 10 mL to produce a yellow solid powder, stable in air, which was filtered off and characterised as L.
Yield: 67.6%.
MALDI-TOF-MS: m/z = 625 [LH]+
1H NMR (CDCl3) δ (ppm) = 8.84 (s, 2H, N=C-H); 8.13–6.90 (m, C-H ar); 5.24 (s, 2H, N-CH); 5.09 (s, 4H, OCH2).
Elemental analysis: calculated for C44H36N2O2 (624.76) C, 84.59%; H, 5.81%; N, 4.49%. Found: C, 84.10%; H, 5.81%; N, 4.55%.
IR (cm-1) (L): 3100 (C-H, Ar), 1640 (C=N), 1620, 1600, 1520 and 1500 (C=C, Ar).
Uv-vis (CHCl3), [L] = 1.0 ×10−5 M, λmax 250 nm, 280 nm and 294 nm
Fluorescence Emission (CHCl3); [L] = 1.0 ×10−5: λmax 340 nm (monomer) and 440 nm (excimer).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
E. Bertolo thanks Canterbury Christ Church University Research Grant Fund for financial support. C. Lodeiro thanks University of Vigo INOU-ViCou K914 (Spain) and Scientific PROTEOMASS Association (Ourense-Spain) for financial support, and Xunta de Galicia for the Isidro Parga Pondal Research programme.
References
- Lodeiro, C.; Capelo, J.L.; Mejuto, J.C.; Oliveira, E.; Santos, H.M.; Pedras, B.; Nuñez, C. Light and colour as analytical detection tools: A journey into the periodic table using polyamines to bio-inspired systems as chemosensors. Chem. Soc. Rev. 2010, 39, 2948–2976. [Google Scholar]
- Lodeiro, C.; Pina, F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coordin. Chem. Rev. 2009, 253, 1353–1385. [Google Scholar]
- Bencini, A.; Lippolis, V. 1,10-phenanthroline: A versatile building block for the construction of ligands for various purposes. Coordin. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar]
- Mewis, R.E.; Archibald, S.J. Biomedical applications of macrocyclic ligand complexes. Coordin. Chem. Rev. 2010, 254, 1686–1712. [Google Scholar]
- Pazos, E.; Vázquez, O.; Mascareñas, J.L.; Vázquez, E.M. Peptide-based fluorescent biosensors. Chem. Soc. Rev. 2009, 38, 3348–3359. [Google Scholar]
- Pedras, B.; Santos, H.M.; Fernades, L.; Covelo, B.; Tamayo, A.; Bertolo, E.; Capelo, J.L.; Avilés, T.; Lodeiro, C. Sensing metal ions with twoazomethine-thiophene pincer ligands (NSN): Fluorescence and MALDI-TOF-MS applications. Inorg. Chem. Commun. 2007, 10, 925–929. [Google Scholar]
- Liu, W.; Xu, L.; Sheng, R.; Wang, P.; Li, H.; Wu, S. A Water Soluble “Switching On” fluorescent Chemosenor of Selectivity to Cd2+. Org. Lett. 2007, 9, 3829–3832. [Google Scholar]
- Yson, M.M. Synthesis and characterisation of a N2O2 containing compound with potential application as a sensor for Cu(II) and Zu (II). BSc Thesis, Canterbury Christ Church University, 2007. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).