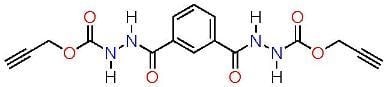
Dipropargyl 2,2'-isophthaloylbis(hydrazinecarboxylate)
Abstract
:Experimental Section
General
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Stieber, F.; Grether, U.; Waldmann, H. Development of the traceless phenylhydrazide linker for solid-phase syntesis. Chem. Eur. J. 2003, 9, 3270–3281. [Google Scholar] [CrossRef] [PubMed]
- Kraatz, U.; Kraemer, W.; Adersch, W.; Turberg, A.; Mencke, N. Fluorobutenic acid hydrazides. Patent DE19530079, 1997. [Google Scholar]
- Skoda-Földes, R.; Szarka, Z.; Kollár, L.; Dinya, Z.; Horváth, J.; Tuba, Z. Synthesis of N-Substituted Steroidal Hydrazides in Homogeneous Catalytic Hydrazinocarbonylation Reaction. J. Org. Chem. 1999, 64, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Kücükgüzel, S.G.; Rollas, S.; Kücükgüzel, I.; Kiraz, M. Synthesis and antimycobacterial activity of some coupling products from 4-aminobenzoic acid hydrazones. Eur. J. Med. Chem. 1999, 34, 1093–1100. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.-Y.; Yi, Y.-P.; Xiang, J.-F.; Chen, C.-F.; Wan, L.-J.; Shuai, Z.-G. Helical Molecular Duplex Strands: Multiple Hydrogen-Bond-Mediated Assembly of Self-Complementary Oligomeric Hydrazide Derivatives. J. Org. Chem. 2007, 72, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.J.; de Parradi, A. All kinds of reactivity: Recent breakthroughs in metal-catalyzed alkyne chemistry. Angew. Chem. Int. Ed. Engl. 2009, 48, 4679–4682. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from af Good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Díaz, D.D.; Punna, S.; Holzer, P.; McPherson, A.K.; Sharpless, K.B.; Fokin, V.V.; Finn, M.G. Click chemistry in materials synthesis. 1. Adhesive polymers from copper-catalyzed azide-alkyne cycloaddition. J. Polym. Sci. Polym. Chem. 2004, 42, 4392–4403. [Google Scholar]
- See Supplementary Files.
- Plusquellec, D.; Roulleau, F.; Lefeuvre, M.; Brown, E. A new synthesis of carboxylic and carbonic acid using phase transfer reactions. Tetrahedron 1988, 44, 2471–2476. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth- Heinemann: Oxford, UK, 1996. [Google Scholar]

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huber, T.; Díaz, D.D. Dipropargyl 2,2'-isophthaloylbis(hydrazinecarboxylate). Molbank 2010, 2010, M701. https://doi.org/10.3390/M701
Huber T, Díaz DD. Dipropargyl 2,2'-isophthaloylbis(hydrazinecarboxylate). Molbank. 2010; 2010(4):M701. https://doi.org/10.3390/M701
Chicago/Turabian StyleHuber, Thimo, and David D. Díaz. 2010. "Dipropargyl 2,2'-isophthaloylbis(hydrazinecarboxylate)" Molbank 2010, no. 4: M701. https://doi.org/10.3390/M701
APA StyleHuber, T., & Díaz, D. D. (2010). Dipropargyl 2,2'-isophthaloylbis(hydrazinecarboxylate). Molbank, 2010(4), M701. https://doi.org/10.3390/M701