Abstract
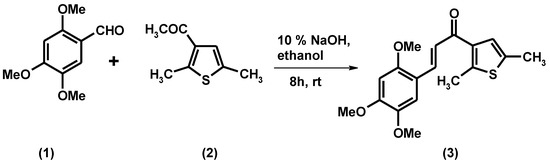
The title compound, 1-(2,5-dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one (3) was synthesized in high yield by an aldol condensation reaction of 3-acetyl-2,5-dimethythiophene and 2,4,5-trimethoxybenzaldehyde in methanolic NaOH at room temperature. Its structure was fully characterized by elemental analysis, IR, 1H NMR, 13C NMR and EI-MS spectral data.
α,β-Unsaturated ketones are biogenic precursors [1] of flavonoids in higher plants, they are also known as chalcones. They display a wide variety of pharmacological properties, including antitumor [2], antibacterial [3], antiviral [4], anti-inflammatory [5], antiulcerative [6] and hepatoprotective activities [7]. Chemically, they consist of either aromatic groups or alkyl groups with an unsaturated chain. Cyclizations of chalcones give pyrazolines, thiazines, or pyrimidines which can show dramatically increased biological activity. On the basis of these aspects, in this paper we are reporting a novel chalcone from 3-acetyl-2,5-dimethythiophene and 2,4,5-trimethoxybenzaldehyde.
A solution of 3-acetyl-2,5-dimethythiophene (0.38 g, 0.0025 mol) and 2,4,5-trimethoxybenzaldehyde (0.49 g, 0.0025 mol) in ethanolic solution of NaOH (6 g in 10 mL of ethanol) was stirred for 16 h at room temperature. The solution was poured onto ice-cold water of pH ~ 2 (pH adjusted by HCl). The separated solid was filtered off, washed several times with a saturated solution of NaHCO3 and left to dry. The residual was recrystallized from methanol/chloroform.
Light-yellow solid: yield: 72%; m.p. 107–108 °C
EI-MS m/z (rel. int.%): 334 (61) [M + 1]+
IR (KBr) vmax cm-1: 3016 (Ar-H), 2924 (C-H), 1642 (C=O), 1572(C=C)
1H NMR (600 MHz, CDCl3)(δ/ppm): 8.01 (d, C=CH, J = 15.6 Hz), 7.26 (s, 1H, CHaromatic), 7.20 (d, C=CH, J = 15.6Hz), 7.08 (s, CHaromatic), 6.51 (s, 1H, 4-CH thiophene), 3.94 (s, OCH3), 3.73 (s, OCH3), 3.62 (s, OCH3), 2.44 (s, 3H, CH3), 2.17 (s, 3H, CH3).
13CNMR (150 MHz, CDCl3) δ: 187.22, 154.44, 152.18, 146.21, 143.11, 138.89, 137.16, 135.08, 126.06, 123.10, 115.45, 111.06, 96.70, 56.49, 56.35, 56.04, 15.80, 15.08.
Anal. calc. for C18H20SO4: C, 65.04, H, 6.06; Found: C, 64.98, H, 5.97.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the deanship of scientific research for the financial support of this work via Grant No. (3-045/430).
References
- Lin, M.; Zhou, Y.; Flavin, M.T.; Zhou, L.; Nie, W.; Chen, F. Chalcones and flavonoids as anti-Tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. [Google Scholar] [CrossRef]
- Wu, X.; Tiekink, E.R.T.; Kostetski, I.; Kocherginsky, N.; Tan, A.L.C.; Khoo, S.B.; Wilairat, P.; Go, M.L. Antiplasmodial activity of ferrocenyl chalcones: Investigations into the role of ferrocene. Eur. J. Pharm. Sci. 2006, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.Y.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Lee, K.H. Antitumor agents. Part 202: Novel 2′-amino chalcones: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2000, 10, 699–701. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, S.A.; Korbad, B.L.; Nile, S.H.; Khobragade, C.N. Synthesis and biological evaluation of β-chloro vinyl chalcones as inhibitors of TNF-α and IL-6 with antimicrobial activity. Eur. J. Med. Chem. 2010, 45, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.C.; Bariwal, J.B.; Upadhyay, K.D.; Naliapara, Y.T.; Joshi, S.K.; Pannecouque, C.C.; Clercq, E.D.; Shah, A.K. Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. Tetrahedron Lett. 2007, 48, 8472–8474. [Google Scholar] [CrossRef]
- Vogel, S.; Barbic, M.; Jurgenliemk, G. Heilmann, Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Forejtnikova, H.; Lunerova, K.; Kubinova, R.; Jankovska, D.; Marek, R.; Kares, R.; Suchy, V.; Vondracek, J.; Machala, M. Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology 2005, 208, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Kadota, S. Neosappanone A, a xanthine oxidase (XO) inhibitory dimeric methanodibenzoxocinone with a new carbon skeleton from Caesalpinia sappan. Tetrahedron Lett. 2004, 45, 8519–8522. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).