Abstract
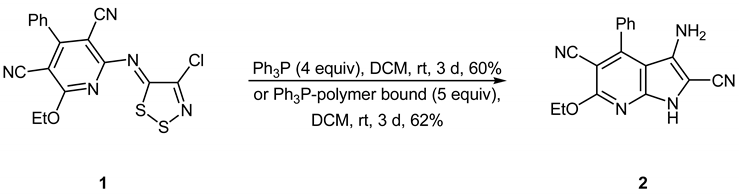
(Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile 1, when treated with either triphenylphosphine (4 equiv.) or polymer bound triphenylphosphine (5 equiv.) in dichloromethane at room temperature for 3 days affords 3‑amino-6-ethoxy-4-phenyl-1H-pyrrolo[2,3-b]pyridine-2,5-dicarbonitrile 2 in 60–62% yields.
Keywords:
pyridine; dithiazole; dithiazolimine; azaindole; indole; heterocycle; polymer bound; solid support Neutral 1,2,3-dithiazoles, readily prepared from 4,5-dichloro-1,2,3-dithiazolium chloride 2 commonly known as Appel’s salt [], are useful precursors to often difficult to access cyano substituted heteroarenes [,,,]. Recently, we demonstrated the unexpected conversion of 2-(4-chloro-5H-1,2,3-dithiazolylideneamino)benzonitriles into 3-aminoindole-2-carbonitriles using triphenyl-phosphine [].
During this study, we treated (Z)-2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile 1 [] with triphenylphosphine (4 equiv.) in dichloromethane at room temperature for 3 days and obtained some triphenylphosphine sulfide (93%) and 3‑amino-6-ethoxy-4-phenyl-1H-pyrrolo[2,3-b]pyridine-2,5-dicarbonitrile 2 in 60% yield. By using polymer bound triphenylphosphine (5 equiv.), the chromatographic separation of the triphenylphosphine sulfide could be avoided and the desired pyrrolo[2,3-b]pyridine was isolated by simple filtration in 62% yield.

Experimental
Anhydrous Na2SO4 was used for drying organic extracts and volatiles were removed under reduced pressure. The reaction mixture and column eluents were monitored by TLC, using commercial glass backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The technique of dry flash chromatography was used, using Merck Silica Gel 60 (less than 0.063 mm). Melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler-Hotstage Microscope apparatus. IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer with Pike Miracle Ge ATR accessory and strong, medium and weak peaks are represented by s, m and w, respectively. 1H NMR spectrum was recorded on a Bruker Avance 300 machine (at 300 MHz). Deuterated chloroform was used for deuterium lock and the signals are referenced to the residual undeuterated solvent peak. Low resolution (EI) mass spectrum was recorded on a Shimadzu Q2010 GCMS with a direct inlet probe. Microanalysis was performed at London Metropolitan University.
3-Amino-6-ethoxy-4-phenyl-1H-pyrrolo[2,3-b]pyridine-2,5-dicarbonitrile (2)
(a) Using free triphenylphosphine. To a stirred solution of (Z)-2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile 1 (100 mg, 0.25 mmol) in DCM (4 mL) at ca. 20 °C and protected with a CaCl2 drying tube, was added triphenylphosphine (262 mg, 1 mmol, 4 equiv.). The mixture was then allowed to stir at ca. 20 °C for 3 days, until no starting materials remained (TLC). The reaction mixture was then adsorbed onto silica and chromatography (hexane/DCM, 5:5) gave triphenylphosphine sulfide (136.7 mg, 93%) as white needles, mp 161–162 °C (from cyclohexane), identical to an authentic sample. Further elusion (hexane/DCM, 2:8) gave the title compound 2 (45.5 mg, 60%) as yellow prisms, mp 191–192 °C (from cyclohexane/EtOH); (Found: C, 67.2; H, 4.4; N, 23.0. C17H13N5O requires C, 67.3; H, 4.3; N, 23.1%); λmax (DCM)/nm 243 (log ε 3.21), 276 (3.46), 314 inf (2.80), 328 (2.93), 372 inf (3.39); vmax/cm-1 3345m (NH), 3231m (NH2), 2234m (C≡N), 2203s (C≡N), 1587s, 1580s, 1516m, 1489m, 1472m, 1381s, 1315s, 1180m, 1159m, 1024m, 924m, 868m, 793m, 772m, 741s, 702s; δH (300 MHz; CDCl3) 8.31 (1H, br s, NH), 7.59–7.56 (3H, m, Ph H), 7.52–7.48 (2H, m, Ph H), 4.51 (2H, q, J 7.1, CH2), 3.74 (2H, br s, NH2), 1.47 (3H, t, J 7.1, CH3); δC (75 MHz, CDCl3) 163.7, 152.6, 146.0, 138.2, 133.1, 130.4 (Ph CH), 129.2 (Ph CH), 128.3 (Ph CH), 115.2 (C≡N), 113.8 (C≡N), 103.1, 91.2, 87.2, 63.8 (CH2), 14.3 (CH3); m/z (EI) 303 (M+, 84%), 275 (100), 247 (11), 219 (12), 194 (9), 165 (12), 140 (70), 51 (5).
(b) Using polymer bound triphenylphosphine. To a stirred solution of (Z)-2-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile 1 (100 mg, 0.25 mmol) in DCM (4 mL) at ca. 20 °C and protected with a CaCl2 drying tube, was added triphenylphosphine polymer bound (328 mg, 1.25 mmol, 5 equiv.). The mixture was then allowed to stir at ca. 20 °C for 3 days, until no starting materials remained (TLC). Filtration of the reaction to remove the polymer, gave 3‑amino-6-ethoxy-4-phenyl-1H-pyrrolo[2,3-b]pyridine-2,5-dicarbonitrile (2) (47 mg, 62%) as yellow prisms mp 191–192 °C (from cyclohexane/EtOH).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors wish to thank the Cyprus Research Promotion Foundation [Grant No. ΤΕΧΝΟΛΟΓΙΑ/ΘΕΠΙΣ/0308(ΒΕ)/08] and the following organisations in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute and the Ministry of Agriculture. Furthermore we thank the A.G. Leventis Foundation for helping to establish the NMR facility in the University of Cyprus.
References and Notes
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reactions of tetracyanoethylene oxide with 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1998, 2505–2509. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Conversion of a 1,2,3-dithiazole into a 3H-pyrrole-3-thione and a 3H-pyrrol-3-ylidenephosphorane. J. Chem. Soc. Perkin Trans. 1 1998, 2765–2769. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Reactions of 1,2,3-dithiazoles with halogenated malononitriles. J. Chem. Soc. Perkin Trans. 1 2002, 1236–1241. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Michaelidou, S.S. 1,2,3-Dithiazole chemistry in heterocyclic synthesis. Arkivoc 2006, 7, 207–223. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Kalogirou, A.S.; Koutentis, P.A. The preparation of dicyano-1,3,4-thiadiazole and tricyanothiazole via 1,2,3-dithiazole chemistry. Tetrahedron 2009, 65, 9967–9972. [Google Scholar] [CrossRef]
- Michaelidou, S.S.; Koutentis, P.A. The conversion of 2-(4-chloro-5H-1,2,3-dithiazolylidene-amino)benzonitriles into 3-aminoindole-2-carbonitriles using triphenylphosphine. Tetrahedron 2009, 65, 8428–8433. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).