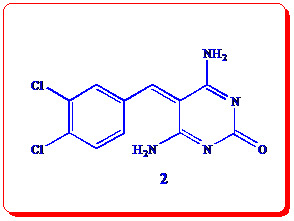
4,6-Diamino-5-(3,4-dichlorobenzylidene)pyrimidin-2(5H)-one
Abstract
:
Antibacterial Activity
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Grivsky, E.M.; Lee, S.; Siyal, C.W.; Duch, D.S.; Nichol, C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimetoxibencil)-5-methylpyrido [2,3-d]Pyrimidine. J. Med. Chem. 1980, 23, 237–329. [Google Scholar] [CrossRef]
- Gossnitzer, E.; Feierl, G.; Wagner, U. Synthesis, structure investigations, and antimicrobial activity of selected s-tran-6-aril-4-isopropyl-2-2[2-[(E)-1-phenylalkylidene]-(E)-hydrazino]-1,4-dihydropyrimidine hydrochlorides. Eur. J. Pharm. Sci. 2002, 15, 49–61. [Google Scholar] [CrossRef]
- Ram, V.J.; Haque, N. Synthesis of functionalized pyrazolo[3,4-d]pyrimidine as potential leishmanicides. Indian J. Chem. 1995, 34B, 521–524. [Google Scholar]
- Calis, U.; Koksal, M. Synthesis and evaluation of anticonvulsant activities of some new aryl-hexahydropyrimidine-2,4-dione. Arzneim.-Forsch/Drug Res. 2001, 51, 523–528. [Google Scholar]
- Saladino, R.; Ciambecchini, U.; Maga, G.; Mastromarino, P.; Conti, C.; Botta, M. A new and efficient synthesis of substituted 6-[(2’-dialkylamino)ethyl]pyrimidine and 4-N,N-dialkyl-6-vinylcytosine derivatives and evaluation of their anti-Rubella activity. Bioorg. Med. Chem. 2002, 10, 2143–2153. [Google Scholar] [CrossRef]
- San-Felix, A.; Velazquez, S.; Perez-Perez, M.J.; Balzarini, J.; De Clercq, E.; Canarasa, M.J. Novel series of TSAO-T derivatives. Synthesis and anti-HIV-1 activity of 4-, 5-. and 6- substituted pyrimidine analogues. J. Med. Chem. 1994, 37, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Vanden Eynde, J.J.; Audiart, N.; Canonne, V.; Michel, S.; van Haverbeke, Y.; Kappe, C.O. Synthesis and Aromatization of Dihydropyrimidines Structurally Related to Calcium Channel Modulators of the Nifedipine-Type. Heterocycles 1997, 45, 1967–1978. [Google Scholar]
- Kumar, R.; Nath, M.; Tyrrell, D.L. Design and synthesis of novel 5-substituted acyclic pyrimidine nucleosides as potent and selective inhibitor of hepatitis B virus. J. Med. Chem. 2002, 45, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bai, Y. Catalysis of the Biginelli Reaction by Ferric and Nickel Chloride Hexahydrates. One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones. Synthesis 2002, 466–470. [Google Scholar] [CrossRef]
- Hazarkhani, H.; Karimi, B. N-Bromosuccinimide as an Almost Neutral Catalyst for Efficient Synthesis of Dihydropyrimidinones Under Microwave Irradiation. Synthesis 2004, 1239–1242. [Google Scholar]
- Lobo, G.; Charris, J.; Valderrama, M.; Taddei, A. 4,6-Diamino-5-[4-(dimethylamino)benzylidene]pyrimidin-2(5H)-one. Molbank 2009, 2009, M615. [Google Scholar] [CrossRef]
- Lobo, G.; Charris, J.; Valderrama, M.; Romero, J.; Castelli, C.; Taddei, A. 4,6-Diamino-5-(4-methylbenzylidene]pyrimidin-2(5H)-one. Molbank 2010, 2010, M653. [Google Scholar] [CrossRef]
- Aydin, F. Synthesis of 4-[4-(dimethylamino)phenyl)]-5-acethyl-6-phenyl-3,4-dihydropyrimidin-2-(1H)thione. Molbank 2006, 2006, M468. [Google Scholar] [CrossRef]
- Tietze, L.F.; Beifus, U.; Trost, B.M.; Fleming, I.; Heathcock, C.H. Comprehensive Organic Synthesis; Pergamon Press: Oxford, UK, 1991; Volume 2, Chapter 1.11; p. 341. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lobo, G.; Charris, J.; Charris, K.; Romero, J.; Taddei, A. 4,6-Diamino-5-(3,4-dichlorobenzylidene)pyrimidin-2(5H)-one. Molbank 2010, 2010, M693. https://doi.org/10.3390/M693
Lobo G, Charris J, Charris K, Romero J, Taddei A. 4,6-Diamino-5-(3,4-dichlorobenzylidene)pyrimidin-2(5H)-one. Molbank. 2010; 2010(3):M693. https://doi.org/10.3390/M693
Chicago/Turabian StyleLobo, Gricela, Jaime Charris, Katiuska Charris, Jesús Romero, and Antonieta Taddei. 2010. "4,6-Diamino-5-(3,4-dichlorobenzylidene)pyrimidin-2(5H)-one" Molbank 2010, no. 3: M693. https://doi.org/10.3390/M693
APA StyleLobo, G., Charris, J., Charris, K., Romero, J., & Taddei, A. (2010). 4,6-Diamino-5-(3,4-dichlorobenzylidene)pyrimidin-2(5H)-one. Molbank, 2010(3), M693. https://doi.org/10.3390/M693