Abstract
The title compound, 5-[(3,5-dimethyl-1-phenyl-1H-pyrazol-4-yl)methylene]-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione, has been synthesized by condensation of 1,3-diethyl-2-thiobarbituric acid and 3,5-dimethyl-1-phenylpyrazole-4- carbaldehyde in ethanol in the presence of pyridine. The structure of this new compound was confirmed by elemental analysis, IR, 1H-NMR, 13C-NMR and EI-MS spectral analysis.
The Knoevenagel condensation of aldehydes with active methylene compounds is an important and widely employed method for donor-acceptor chromophore formation in organic synthesis [1] with numerous applications in the synthesis of fine chemicals such as photoelectronics [2], photophotonics, photodynamic therapy [3], electrochemical sensing [4], optical limiting [5], Langmuir film and photoinitiated polymerization [6]. The donor acceptor chromophores are also applicable in the field of biomedicinal chemistry. Due to the wide application of donor-acceptor chromophores, the authors have undertaken the synthesis of a novel donor-acceptor chromophore.
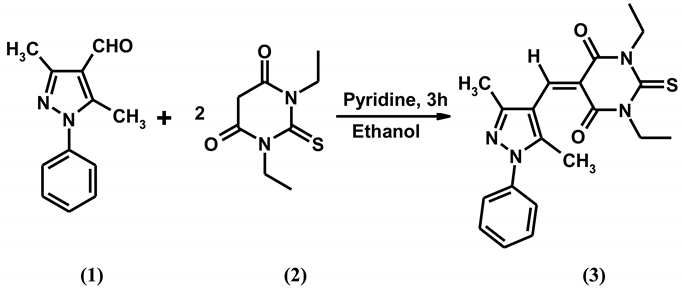

A mixture of 1,3-diethyl-2-thiobarbituric acid (1) (1.0 g, 0.005 mol), 3,5-dimethyl-1-phenylpyrazole-4-carbaxaldehyde (2) (1.0 g, 0.005 mol) and a few drops of pyridine in anhydrous ethanol (15 mL) was refluxed at 80 °C for 3 h with continuous stirring. Progress of the reaction was monitored by TLC. After completion of the reaction, the solution was cooled. The heavy precipitate thus obtained was collected by filtration and purified by recrystallization from methanol/chloroform to give the title compound (3).
Yield: 78%; m.p. 177–178 °C.
EI-MS m/z (rel. int.%): 383 (62) [M+1]+.
IR (KBr) vmax cm-1: 2979 (C-Haromatic), 2926 (C-Haliphatic) 1694 (C=O), 1568 (C=C), 1194 (C-N), 1172 (C=S).
1H-NMR (600 MHz, CDCl3) δ: 8.56 (s, C=CH), 7.52 (d, J = 3.6 Hz, CHaromatic), 7.50 (d, J = 1.8 Hz, CHaromatic), 7.45 (dd, J = 3.0, 2.8 Hz, CHaromatic), 7.43 (dd, J =1.2, 1.8 Hz , CHaromatic), 7.24 (dd, J =1.4, 1.6 Hz, CHaromatic) 4.56 (t, J = 3.6 Hz, CH3-CH2-N), 2.37 (s, -CH3), 2.28 (s, -CH3), 1.34 (q, J = 6.0 Hz, CH3-CH2-N),
13C-NMR (600 MHz, CDCl3) δ: 178.94, 160.84, 158.29, 152.91, 150.40, 145.19, 138.46, 129.34, 128.69, 125.04, 117.63, 114.45, 43.92, 43.50, 14.63, 13.55, 12.70, 12.43.
Anal. calc. for C20H22O2N4S: C, 62.82, H, 5.75, N, 14.65. Found: C, 62.76, H, 5.55, N, 14.58.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank the Chemistry Department, King Abdul Aziz University, Jeddah, Saudi Arabia for providing the research facilities.
References and Notes
- Asiri, A.M. Synthesis and characterization of dyes exemplified by 2-arylidene-dicyanomethyleneindane. Dyes Pigm. 1999, 42, 209–213. [Google Scholar] [CrossRef]
- Jiang, L.; Chang, Q.; Ouyang, Q.; Liu, H.; Wang, Y.; Zhang, X.; Song, Y.; Li, Y. Fabrication and nonlinear optical properties of an ultrathin film with acceptor–donor periodically overlapping structure. Chem. Phys. 2006, 324, 556–562. [Google Scholar] [CrossRef]
- Wu, C.; Tretiak, S.; Chernyak, V.Y. Excited states and optical response of a donor–acceptor substituted polyene: A TD-DFT study. Chem. Phy. Lett. 2007, 433, 305–311. [Google Scholar] [CrossRef]
- Chandrassekarn, Y.; Dutta, G.K.; Kanth, R.B.; Patil, S. Tetrahydroquinoxaline based squaraines: Synthesis and photophysical properties. Dyes Pigm. 2009, 83, 162–167. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Zhang, X.; Liu, Z.; Wan, X.; Tian, J.; Wang, T.; Chen, Y. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 2009, 47, 3113–3121. [Google Scholar] [CrossRef]
- Bosch, P.; Peinado, C.; Martin, V.; Catalina, F.; Corrales, T. Fluorescence monitoring of photoinitiated polymerization reactions: Synthesis, photochemical study and behaviour as fluorescent probes of new derivatives of 4′-dimethylaminostyryldiazines. J. Photochem. Photobio. A: Chem. 2006, 180, 118–129. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).