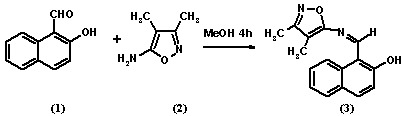
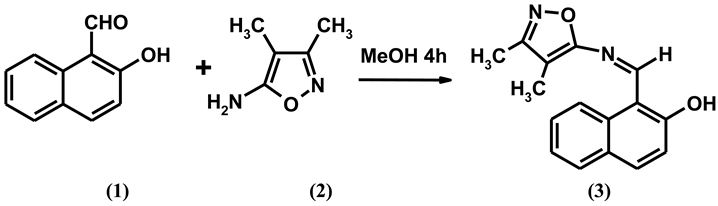
1-{[(3,4-Dimethylisoxazol-5-yl)imino]methyl}-2-naphthol
Abstract
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Panneerselvam, P.; Rather, B.A.; Reddy, D.R.S.; Kumar, N.R. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6,8-dibromo-2-phenylquinazolin-4(3H)-ones. Eur. J. Med. Chem. 2009, 44, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, J.; Bai, J. Synthesis and antimicrobial activity of the Schiff base from chitosan a; nd citral. Carbohyd. Res. 2009, 344, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2008, 43, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z. Crystal structures, antioxidation and DNA binding properties of Eu(III) complexes with Schiff-base ligands derived from 8-hydroxyquinoline-2-carboxyaldehyde and three aroylhydrazines. J. Inorg. Biochem. 2009, 103, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Akhter, Z.; Yameen, S.; Siddiqi, H.M.; Mirza, B.; Rifat, A. Synthesis and biological evaluations of some Schiff-base esters of ferrocenyl aniline and simple aniline. J. Orgnometa. Chem. 2009, 694, 2198–2203. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 1-{[(3,4-Dimethylisoxazol-5-yl)imino]methyl}-2-naphthol. Molbank 2010, 2010, M665. https://doi.org/10.3390/M665
Asiri AM, Khan SA. 1-{[(3,4-Dimethylisoxazol-5-yl)imino]methyl}-2-naphthol. Molbank. 2010; 2010(1):M665. https://doi.org/10.3390/M665
Chicago/Turabian StyleAsiri, Abdullah Mohamed, and Salman A. Khan. 2010. "1-{[(3,4-Dimethylisoxazol-5-yl)imino]methyl}-2-naphthol" Molbank 2010, no. 1: M665. https://doi.org/10.3390/M665
APA StyleAsiri, A. M., & Khan, S. A. (2010). 1-{[(3,4-Dimethylisoxazol-5-yl)imino]methyl}-2-naphthol. Molbank, 2010(1), M665. https://doi.org/10.3390/M665