Abstract
A new 2-(4-propyloxyphenyl)benzothiazole was synthesized and its IR, 1H and 13C NMR and MS spectroscopic data are presented.
Heterocyclic molecules have received overwhelming responses owing to their diversified molecular design and remarkable optical [1,2,3], liquid crystalline [4] and electronic properties [5]. 2-Substitued benzothiazole has emerged in its usage as a core structure in calamitic liquid crystals. It has been reported that liquid crystalline compounds incorporating a benzothiazole ring exhibit good hole-transporting properties with a low ionization potential, making them of potential interest as hole-transporting materials in organic light-emitting devices (OLEDs) [6,7,8]. In view of the potential applications of these compounds, we are prompted to generate the derivatives by introducing different substituents into the existing skeleton of the molecule [9,10].
Preparation of Benzothiazole 1
2-Aminothiophenol (5.01 g, 40 mmol) and 4-hydroxybenzaldehyde (4.88 g, 40 mmol) in absolute ethanol (40 mL) was heated under reflux for 6 hours. The reaction mixture was subsequently cooled to room temperature, then distilled water (60 mL) was added slowly until the mixture turned cloudy. It was kept overnight at about 20 °C and the solid formed was filtered and washed with cold ethanol:water (1:1.5) and dichloromethane.
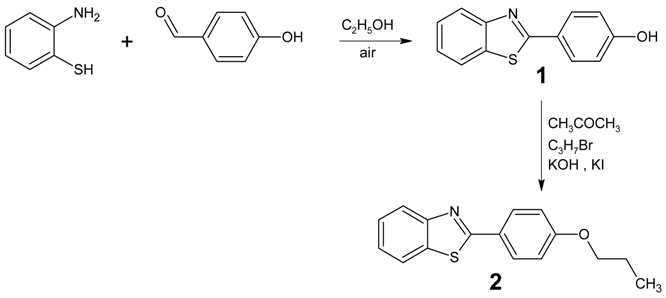
Preparation of Benzothiazole 2
In analogy to a recently published procedure [11], benzothiazole 1 (4.55 g, 20 mmol) in acetone (40 ml), was added to a solution of potassium hydroxide (1.12 g, 20 mmol) in distilled water (5 ml). This was followed by addition of a small amount of potassium iodide into the mixture. The reaction mixture was heated under reflux for an hour with stirring. 1-Bromopropane (3.07 g, 25 mmol) was then added to the flask and reflux was continued for 20 hours. The solid obtained was repeatedly recrystallized from absolute ethanol whereupon the pure compound was isolated as a white solid (2.53 g, 47%).
Melting point: 113.3 °C.
EI-MS m/z (rel. int. %): 269 (42) [M+], 227 (100), 198 (12), 108 (10), 69 (6).
IR (KBr, cm-1): 2958, 2935 (C-H aliphatic); 1600 (C=N); 1259, 1064 (C-O ether).
1H NMR (400 MHz, CDCl3): δ/ppm 1.0 (t, J = 7.4 Hz, 3H, CH3-), 1.8 (qt, J = 7.0, 7.4 Hz, 2H, CH3-CH2-CH2-O-), 4.0 (t, J = 6.6 Hz, 2H, -CH2-O-), 7.0 (d, J = 8.8 Hz, 2H, Ar-H), 7.3 (dd, J = 1.9, 7.8 Hz, 1H, Ar-H), 7.4 (dd, J = 2.0, 8.0 Hz, 1H, Ar-H), 7.8 (d, J = 8.0 Hz, 1H, Ar-H), 8.0 (m, 3H, Ar-H).
13C NMR (400 MHz, CDCl3): δ/ppm 167.9 (C=N), 161.5, 154.2, 134.8, 129.1, 126.2, 126.1, 124.7, 122.8, 121.5, 114.8 for aromatic carbons, 69.7 (-O-CH2-), 30.9 [-(CH2)-CH2O-], 22.5 (-CH3).
Elemental analysis: Calculated for C16H15NOS: C, 71.34%, H, 5.61%, N, 5.20%; Found: C, 71.41%, H, 5.50%, N, 5.13%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The author (S.T. Ha) would like to thank Universiti Tunku Abdul Rahman (UTAR) for the financial support through UTAR Research Fund (Vote No. 6200/H02), and the Malaysia Toray Science Foundation (UTAR Vote No. 4359/000) for funding this project. T.M. Koh would like to acknowledge UTAR for the award of the research and teaching assistantships.
References and Notes
- Chen, X.L.; Jenekhe, A. Macromolecules 1996, 29, 6189–6192.
- Chou, S.S.P.; Sun, D.J.; Lin, H.C.; Yang, P.K. Chem. Commun. 1996, 1045–1046.
- Raimundo, J.M.; Blanchard, P.; Ledoux-Rax, I.; Hierle, R.; Michaux, L.; Roncali, J. Chem. Commun. 2000, 1597–1598.
- Lee, C.H.; Yamamoto, T. Mol. Cryst. Liq. Cryst. 2001, 363, 77–84.
- Maruyama, T.; Suganuma, H.; Yamamoto, T. Synthetic Metals 1995, 74, 183–185.
- Funahashi, M.; Hanna, J.I. Jpn J. Appl. Phys. 1996, 35, L703.
- Funahashi, M.; Hanna, J.I. Phys. Rev. Lett. 1997, 78, 2184.
- Funahashi, M.; Hanna, J.I. Mol. Cryst. Liq. Cryst. 1997, 304, 429.
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Boey, P.L.; Yip, F.W.; Ong, S.T.; Ong, L.K. Mol. Cryst. Liq. Cryst. 2009, in press.
- Ha, S.T.; Koh, T.M.; Yeap, G.Y.; Lin, H.C.; Beh, J.K.; Win, Y.F.; Boey, P.L. Chin. Chem. Lett. 2009, in press.
- Ha, S.T.; Ong, L.K.; Wong, J.P.W.; Win, Y.F.; Koh, T.M. Molbank 2009, 1, M598.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).