Abstract
A new Schiff base 4-[(pyridin-3-ylmethylene)amino]phenyloctadecanoate was synthesized and its IR, 1H NMR, 13C NMR and MS spectroscopic data are presented.
Schiff bases have received a considerable amount of attention from many researchers owing to their importance in exhibiting thermochromism and photochromism [1,2,3,4].
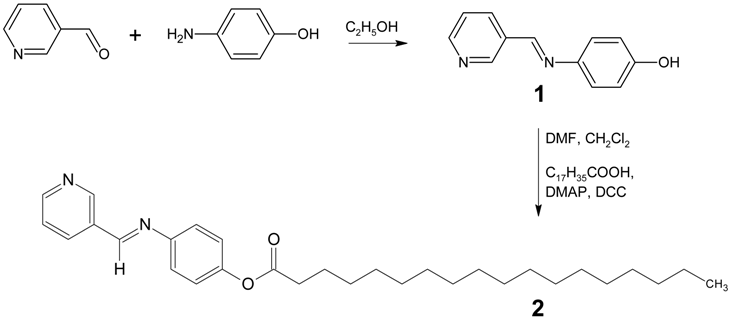
In analogy to a recently published procedure [5,6,7], a solution of 3-pyridinecarbaldehyde (4.28 g, 40 mmol) and 4-aminophenol (4.37g, 40 mmol) in absolute ethanol (70 mL) was heated under reflux for 3 hours. Schiff base 1 thus obtained was recrystallized from absolute ethanol. Then, Schiff base 1 (3.96 g, 20 mmol) in dimethylformamide (DMF) (4 mL), was added into a solution of stearic acid (5.69g, 20 mmol) and 4-dimethylaminopyridine (DMAP) (1.22 g, 10 mmol) in dichloromethane (70 mL). The resulting mixture was stirred in an ice bath. To this solution, N,N’-dicyclohexylcarbodiimide (DCC) (4.12 g, 20 mmol) dissolved in dichloromethane (20 mL) was added dropwise while stirring in the ice bath for an hour. The resulting mixture was subsequently stirred at room temperature for another 3 hours. Then, the reaction mixture was filtered and the excess solvent was removed from the filtrate by evaporation. Recrystallization from absolute ethanol gave the Schiff base 2 as gray solid (3.72g, 40%).
Melting Point: 92.2°C.
MS(EI): M+ (m/z) = 464
IR (KBr, cm-1): 2954, 2916, 2848 (C-H aliphatic); 1754 (C=O ester); 1626 (C=N); 1595, 1499 (C=C aromatic).
1H NMR (400 MHz, CDCl3): δ/ppm 0.89 (3H, t, CH3), 1.23-1.45 {m, 28H, CH3(CH2)14-}, 1.76 (qt, 2H, -CH2CH2COO-), 2.58 (t, 2H, -CH2COO-), 7.13 (d, 2H, Ar-H), 7.25 (d, 2H, Ar-H), 7.41 (dd, 1H, Ar-H), 8.28 (d, 1H, Ar-H), 8.50 (s, 1H, CH=N), 8.71 (dd, 1H, Ar-H), 9.01 (d, 1H, Ar-H).
13C NMR (100 MHz, CDCl3): δ/ppm 172.7 (COO), 157.6 (CH=N), 152.5, 151.4, 149.8, 149.3, 135.3, 132.2, 124.2, 122.7 and 122.2 (aromatic carbons), 34.8 (-CH2COO-), 25.3 (-CH2CH2COO-), 32.3, 30.1, 30.0, 29.9, 29.8, 29.7, 29.5, 25.3 and 23.1 (CH3(CH2)14-), 14.5 (CH3).
Elemental analysis: Calculated for C30H44N2O2: C, 77.54%, H, 9.54%, N, 6.03%; Found: C, 77.65%, H, 9.59%, N, 5.92%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors would like to thank Universiti Tunku Abdul Rahman and Universiti Sains Malaysia for financial support and research facilities.
References and Notes
- Hadjoudis, E.; Vittorakis, M.; Moustakali-Mavridis, I. Tetrahedron 1987, 43, 1345–1360. [CrossRef]
- Hadjoudis, E.; Rontoyianni, A.; Ambroziak, K.; Dziembowska, T.; Mavridis, I.M. J. Photochem. Photobiol. A: Chem. 2004, 162, 521–530. [CrossRef]
- Oshima, A.; Momotake, A.; Arai, T. J. Photochem. Photobiol. A: Chem. 2004, 162, 473–479.
- Suzuki, T.; Arai, T. Chem. Lett. 2001, 124–125.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2008, (3), M582.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2009, (1), M584.
- Ha, S.T.; Ong, L.K.; Win, Y.F.; Koh, T.M.; Yeap, G.Y. Molbank 2009, (1), M585.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).