Synthesis of 3-[2-(4-Chlorobenzylidene)hydrazino]-3-oxo-N-(4-sulfamoylphenyl)propanamide
Abstract
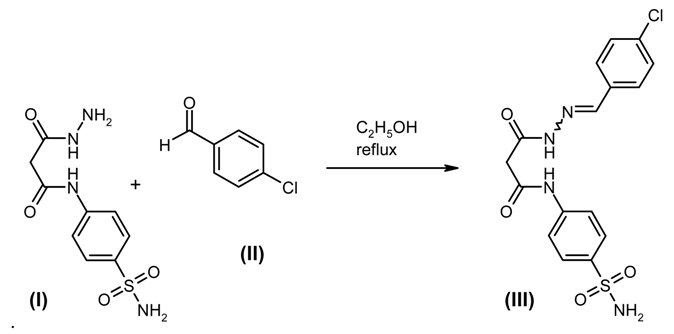
:
- Elemental analysis:
- Calcd for C16H15N4O4SCl (394.5): C, 48.66%; H, 3.80%; N, 14.19%.Found: C, 48.69%; H, 3.76 %; N, 14.21%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References and Notes
- Polanc, S. Recent Application of Hydrazides and Related Compounds for the Synthesis of Heterocycles. Targets Heterocycl. Syst. 1999, 3, 33–91, [Chem. Abstr. 2000, 133, 237877u]. [Google Scholar]
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and Antimicrobial Activity of Some New Hydrazones of 4-Fluorobenzoic Acid Hydrazide and 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Habib, N. S.; Issa, A. S.; Rida, S. M.; Ashour, F. A.; Tawil, G. G. Synthesis of Alkyloxybenzamide Derivatives as Potential Antimicrobial Agents. Pharmazie 1986, 41, 761–764. [Google Scholar] [PubMed]
- Dimmock, J. R.; Vashishtha, S. C.; Stables, J. P. Anticonvulsant Properties of Various Acetylhydrazones, Oxamoylhydrazones and Semicarbazones Derived from Aromatic and Unsaturated Carbonyl Compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef]
- Kalsi, R.; Pande, K.; Bhalla, T. N.; Barthwal, J. P.; Gupta, G. P.; Parmar, S. S. Antiinflammatory Activity of Quinazolinoformazans. J. Pharm. Sci. 1990, 79, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Kucukguzel, S. G.; Mazi, A.; Sahin, F.; Ozturk, S.; Stables, J. Synthesis and Biological Activities of Diflunisal Hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Ergenç, N.; Günay, N. S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Fraga, A. G. M.; Rodrigues, C. R.; Miranda, A. L. P.; Barreiro, E. J.; Fraga, C. A. M. Synthesis and Pharmacological evaluation of novel heterocyclic acylhydrazone derivatives, designed as PAF Antagonists. Eur. J. Pharm. Sci. 2000, 11, 285–290. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D. B.; Gordeuk, V. R.; Richardson, D. R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef]
- Savini, L.; Chiasserini, L.; Travagli, V.; Pellerano, C.; Novellino, E.; Cosentino, S.; Pisano, M. B. New α-heterocyclic hydrazones: evaluation of anticancer, anti-HIV and antimicrobial activity. Eur. J. Med. Chem. 2004, 39, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mort, J.; Pfister, G. Electronic Properties of Polymers; Wiley: New York, 1982. [Google Scholar]
- Döbler, M.; Weder, C.; Neuenschwander, P.; Suter, U.W.; Follonier, S.; Bosshard, C.; Günter, P. Synthesis and Characterization of New Photorefractive Polymers with High Glass Transition Temperatures. Macromolecules 1998, 31, 6184–6189. [Google Scholar] [CrossRef]
- Nam, H.; Kang, D. H.; Kim, J. K.; Park, S. Y. Synthesis of Hole-Transporting Hydrazone Dendrimers. Chem. Lett. 2000, 11, 1298–1299. [Google Scholar] [CrossRef]
- Simokaitiene, J.; Danilevicius, A.; Grigalevicius, S.; Grazulevicius, J. V.; Getautis, V.; Jankauskas, V. Phenotiazinyl-based hydrazones as new hole-transporting materials for electrophotographic photoreceptors. Synthetic Metals 2006, 156, 926–931. [Google Scholar] [CrossRef]
- Naqvi, A.; Shahnawaaz, M.; Rao, A. V.; Seth, D. S.; Sharma, N. K. Synthesis of novel Hydrazino-3-oxo-N-(4-sulfamoylphenyl)-propanamide. Molbank 2009, M586. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naqvi, A.; Shahnawaaz, M.; Rao, A.V.; Seth, D.S.; Sharma, N.K. Synthesis of 3-[2-(4-Chlorobenzylidene)hydrazino]-3-oxo-N-(4-sulfamoylphenyl)propanamide. Molbank 2009, 2009, M597. https://doi.org/10.3390/M597
Naqvi A, Shahnawaaz M, Rao AV, Seth DS, Sharma NK. Synthesis of 3-[2-(4-Chlorobenzylidene)hydrazino]-3-oxo-N-(4-sulfamoylphenyl)propanamide. Molbank. 2009; 2009(1):M597. https://doi.org/10.3390/M597
Chicago/Turabian StyleNaqvi, Arshi, Mohd. Shahnawaaz, Arikatla V. Rao, Daya S. Seth, and Nawal K. Sharma. 2009. "Synthesis of 3-[2-(4-Chlorobenzylidene)hydrazino]-3-oxo-N-(4-sulfamoylphenyl)propanamide" Molbank 2009, no. 1: M597. https://doi.org/10.3390/M597
APA StyleNaqvi, A., Shahnawaaz, M., Rao, A. V., Seth, D. S., & Sharma, N. K. (2009). Synthesis of 3-[2-(4-Chlorobenzylidene)hydrazino]-3-oxo-N-(4-sulfamoylphenyl)propanamide. Molbank, 2009(1), M597. https://doi.org/10.3390/M597



