Synthesis
The synthesis starts from methionine (1) which was protected at the N-terminus using benzyl carbonochloridate. Subsequently, the tbutyl-ester was introduced using a Steglich-type esterification reaction. After methylation resulting in the sulfonium salt 2, the cyclisation reaction was performed using 4-bromobenzaldehyde 3. rac-tert-Butyl 3-(benzyloxycarbonylamino)-2-(4-bromophenyl)-tetrahydrofuran-3-carboxylate (rac-4) was formed in a highly diastereoselective manner as a racemic mixture of the trans-products in a yield of 48 %.
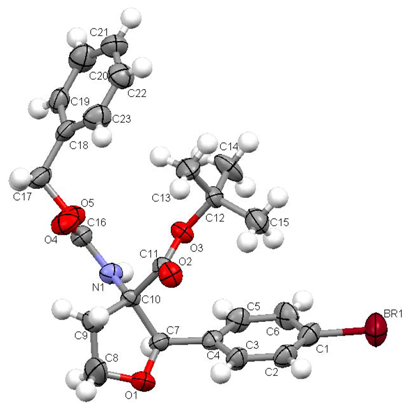
The connectivity and relative configuration of tert-butyl 3-(benzyloxycarbonylamino)-2-(4-bromophenyl)-tetrahydrofuran-3-carboxylate (4) was confirmed by an X-ray structure analysis. Suitable crystals were obtained by recrystallization from MeOH.
tert-Butyl 2-(benzyloxycarbonylamino)-4-(methylthio)butanoate
2-(Benzyloxycarbonylamino)-4-(methylthio)butanoic acid (1, 3.00 g, 10.6 mmol) was dissolved under a nitrogen atmosphere in 50 ml of dry DCM and cooled to 0 °C in an ice bath. To this solution DMAP (108 mg, 0.88 mmol) and tbutanol (1.21 ml, 12.7 mmol) were added. Under vigorous stirring dicyclohexyl carbonate (2.84 g, 13.8 mmol) was slowly added in portions. The mixture was stirred at 0 °C for 2 hours, allowed to warm to room temperature and stirred for additional 12 hours. Precipitated urea was filtered off and washed twice with 25 ml of DCM. The solvent was evaporated under reduced pressure and the crude product was purified by column chromatography on silica gel (EtOAc:PE 30:70, Rf = 0.48) to give the product as colorless oil (1.84 g, 6.02 mmol, 57 %).
MS (CI, NH3): m/z (%) = 284.0 (7) [MH+ - C4H8], 301.1 (52) [MNH4+ - C4H8], 340.1 (15) [MH+], 357.1 (100) [MNH4+].
IR (NEAT) [cm-1]: = 2979, 2917, 1708, 1523, 1455, 1368, 1224, 1149, 1046, 965, 845, 738, 696.
1H-NMR (300 MHz, CDCl3): δ = 1.46 (s, 9 H, 13), 1.82-2.20 (m, 5 H, 14 + 17), 2.41-2.61 (m , 2 H, 15), 4.25-4.44 (m, 1 H, 9), 5.11 (s, 2 H, 5), 5.39 (d, 3JH,H = 7.4, 1 H, 8), 7.28-7.43 (m, 5 H, H-Ar).
13C-NMR (75 MHz, CDCl3): δ = 15.5 (+, 1 C, 17), 28.0 (+, 3 C, 13), 29.9 (-, 1 C, 15), 32.5 (+, 1 C, 14), 53.8 (+, 1 C, 9), 67.0 (+, 1 C, 5), 82.4 (Cquat, 1 C, 12), 128.1 (+, 2 C, 3), 128.2 (+, 1 C, 1) , 128.5 (+, 2 C, 2), 136.3 (Cquat, 1 C, 4), 155.9 (Cquat, 1 C, 7), 171.0 (Cquat, 1 C, 10).
(3-(Benzyloxycarbonylamino)-4-tert-butoxy-4-oxobutyl)dimethylsulfonium iodide (2)
tert-Butyl 2-(benzyloxycarbonylamino)-4-(methylthio)butanoate (3.00 g, 10.6 mmol) was dissolved in 32 ml of methyl iodide (3 ml/mmol) and stirred for three days at room temperature in the dark. The solution was cooled to 0 °C in an ice bath and 32 ml of heptane (3 ml/mmol) were added to precipitate the product. The mixture was kept at 0 °C in the dark for four hours to complete the precipitation. The hygroscopic and light sensitive product was obtained after filtration and washing with ice-cold heptane as a colorless solid (1.39 g, 2.89 mmol, 98%) in analytically pure form.
MP 35-37 °C.
MS (ES, DCM/MeOH + 10 mmol/l NH4OAc): m/z (%) = 354.1 (100) [M+].
IR (NEAT) [cm-1]: = 2980, 2928, 1707, 1518, 1238, 1151, 1047, 740, 698.
1H-NMR (300 MHz, CDCl3): δ = 1.46 (s, 9 H, 13), 2.20-2.49 (m, 2 H, 14), 3.19 (s, 3 H, 17), 3.23 (s, 3 H, 17), 3.59-3.81 (m, 1 H, 15), 3.85-4.02 (m, 1 H, 15), 4.21-4.38 (m, 1 H, 9), 5.10 (s, 2 H, 5), 6.09 (d, 3JH,H = 7.1, 1 H, 8), 7.28-7.47 (m, 5 H, 1 -3).
13C-NMR (75 MHz, CDCl3): δ = 25.7 (+, 1 C, 17), 26.0 (+, 1 C, 17), 28.0 (+, 3 C, 13), 31.9 (-, 1 C, 14), 40.5 (+, 1 C, 15), 53.2 (+, 1 C, 9), 67.2 (+, 1 C, 5), 83.5 (Cquat, 1 C, 12), 128.2 (+, 2 C, 3), 128.3 (+, 1 C, 1) , 128.6 (+, 2 C, 2), 136.3 (Cquat, 1 C, 4), 156.5 (Cquat, 1 C, 7), 169.9 (Cquat, 1 C, 10).
rac-tert-Butyl 3-(benzyloxycarbonylamino)-2-(4-bromophenyl)-tetrahydrofuran-3-carboxylate (rac-4)
In an oven dried Schlenk flask under nitrogen atmosphere (3-(benzyloxycarbonylamino)-4-tert-butoxy-4-oxobutyl)-dimethylsulfonium iodide (
2, 4.68 g, 9.73 mmol, 1.2 eq.) was dissolved in dry acetonitrile (5 ml per 1 mmol sulfonium salt). The colorless solution was cooled to -6 °C and potassium hydroxide (546 mg, 9.73 mmol, 1.2 eq.) followed by
4-bromobenzaldehyde (
4, 1.50 g, 8.11 mmol, 1 eq.) were added. The mixture was stirred at -6 °C for 6 h. After complete consumption of the starting material (checked via TLC, 60:40 PE:diethyl ether, R
f = 0.05) the reaction mixture was quenched with water (4 ml per mmol sulfonium salt) and extracted with diethyl ether (1 x 4 ml/mmol, 2 x 5 ml/mmol sulfonium salt). The combined organic layers were washed with brine solution and dried over MgSO
4. After removal of the solvent under reduced pressure the crude product was purified by flash chromatography [
9] using a 80:20 mixture of PE:diethyl ether (R
f = 0.15) to give a white crystalline solid in 48 % yield (1.85 g, 3.90 mmol).
MP 133-135 °C.
MS (ES, DCM/MeOH + 10 mmol/l NH4OAc): m/z (%) = 476.2 (7) [MH+], 495.2 (100) [MNH4+], 476.1 (100) [M – H+], 536.2 (10) [M + CH3COO-].
IR (NEAT) [cm-1]: = 3334, 2977, 2947, 2860, 1702, 1593, 1524, 1491, 1361, 1247, 1154, 1073, 1011, 986, 827, 790, 750, 697.
1H-NMR (300 MHz, CDCl3): δ = 1.11(s, 9 H, tBu), 2.54-2.81 (m, 2 H, CH2), 4.22-4.37 (m, 2 H, O-CH2), 5.08-5.21 (m, 3 H, CH2–Cbz + CH), 6.00 (bs, 1 H, NH), 7.20 (d, 3JH,H = 7.9, 2 H, CH-Ar), 7.28-7.45 (m, 7 H, CH-Cbz, CH-Ar).
13C-NMR (75 MHz, CDCl3): δ = 27.4 (+, 3 C, CH3-tBu), 35.8 (-, 1 C, CH2), 66.8 (-, 1 C, O-CH2), 67.9 (-, 1 C, CH2-Bzl), 69.5 (Cquat, 1 C, C-NH), 83.0 (Cquat, 1 C, C-tBu), 83.8 (+, 1 C, CH) , 121.7 (Cquat, 1 C, C-Br), 127.7 (+, 2 C, CH-Bzl), 128.2 (+, 2 C, CH-Bzl), 128.3 (+, 1 C, CH-Bzl), 128.6 (+, 2 C, CH-Ar), 131.0 (+, 2 C, CH-Ar), 136.2 (Cquat, 1 C, C-Ar), 136.5 (Cquat, 1 C, C-Ar), 154.6 (Cquat, 1 C, CO-Bzl), 169.9 (Cquat, 1 C, COOtBu).
Elemental analysis calcd. (%) for C23H26BrNO5 (524.49): C 57.99, H 5.50, N 2.94; found: C 58.04, H 5.55, N 2.82.