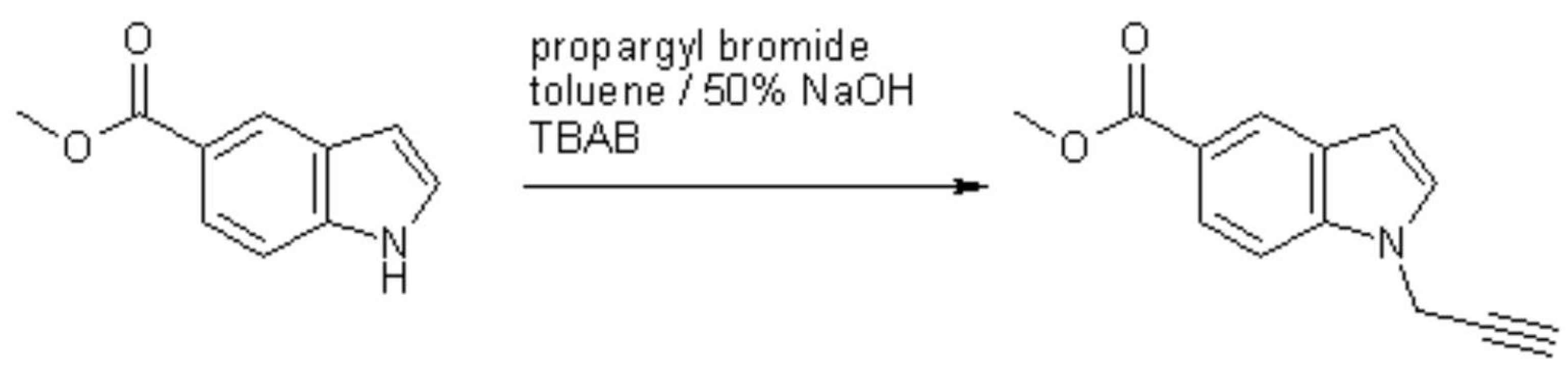
The title compound, which is a useful synthon in Sonogashira cross-coupling reactions, can be prepared by N-alkylation of methyl indole-5-carboxylate with propargyl bromide in toluene / 50% sodium hydroxide under phase-transfer catalysis. Under these conditions [1], the ester group remains unaffected and there is no noticeable alkyne/allene rearrangement [2]. Thus, to a solution of methyl indole-5-carboxylate (1.75 g, 10 mmol) and propargyl bromide (2.23 g, 15 mmol of an 80% solution in toluene) in toluene (30 mL) was added tetrabutylammonium bromide (0.161 g, 0.5 mmol) and 50% aqueous NaOH (6 mL). The two-phase system was stirred vigorously for 6 h at room temperature, then it was diluted with toluene (10 mL) and the phases were separated. The organic layer was washed several times with water and then with brine. It was dried over Na2SO4 and the solvent was removed under reduced pressure. The residue was recrystallised from diisopropyl ether to give the product (1.432 g, 67%) as yellow crystals.
Melting point: 94–96 °C
IR (KBr, cm-1): n 3244, 3111, 2947, 2110, 1688, 1613, 1430, 1316, 1200, 1100, 751, 696.
1H-NMR (CDCl3, 300 MHz): d = 8.41 (d, J4–6 = 1.5 Hz, 1H, 4-H), 7.97 (dd, J6–7 = 8.6 Hz, J4–6 = 1.5 Hz, 1H, 6-H), 7.42 (d, J6-7 = 8.6 Hz, 1H, 7-H), 7.29 (d, J2–3 = 3.3 Hz, 1H, 2-H), 6.64 (d, J2–3 = 3.3 Hz, 1H, 3-H), 4.91 (d, J = 2.6 Hz, 2H, CH2), 3.94 (s, 3H, OCH3), 2.44 (t, J = 2.6 Hz, 1H, C≡CH).
13C-NMR (CDCl3, 75 MHz): d = 168.02, 138.12, 128.59, 128.36, 124.00, 123.22, 121.94, 108.95, 103.53, 77.09, 73.98, 51.81, 35.93.
MS (relative intensity): m/z = 213 (M+, 90%), 198 (14), 182 (100), 154 (83), 127 (34), 115 (23), 77 (16), 63 (20), 51 (12).
Elemental Analysis: Calculated for C13H11NO2 (213.24): C, 73.23%; H, 5.20%; N, 6.57%. Found: C, 73.05%; H, 5.20%; N, 6.35%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Broggini, G.; Bruché, L.; Zecchi, G.; Pilati, T. 1,3-Dipolar cycloadditions to nitrogen-substituted allenes. J. Chem. Soc., Perkin Trans. 1 1990, 533–539. [Google Scholar] [CrossRef]
- For recent examples of alkyne/allene rearrangements of N-propargylindoles, see: Haider, N.; Käferböck, J. Intramolecular [4+2] cycloaddition reactions of indolylalkylpyridazines: synthesis of annulated carbazoles. Tetrahedron 2004, 60, 6495–6507. [Google Scholar] Abbiati, G.; Canevari, V.; Caimi, S.; Rossi, E. Domino addition/annulation of delta-alkynylaldehydes and oxygen nucleophiles. A new entry to [1,4]oxazino[4,3-a]indoles. Tetrahedron Lett. 2005, 46, 7117–7120. [Google Scholar]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.