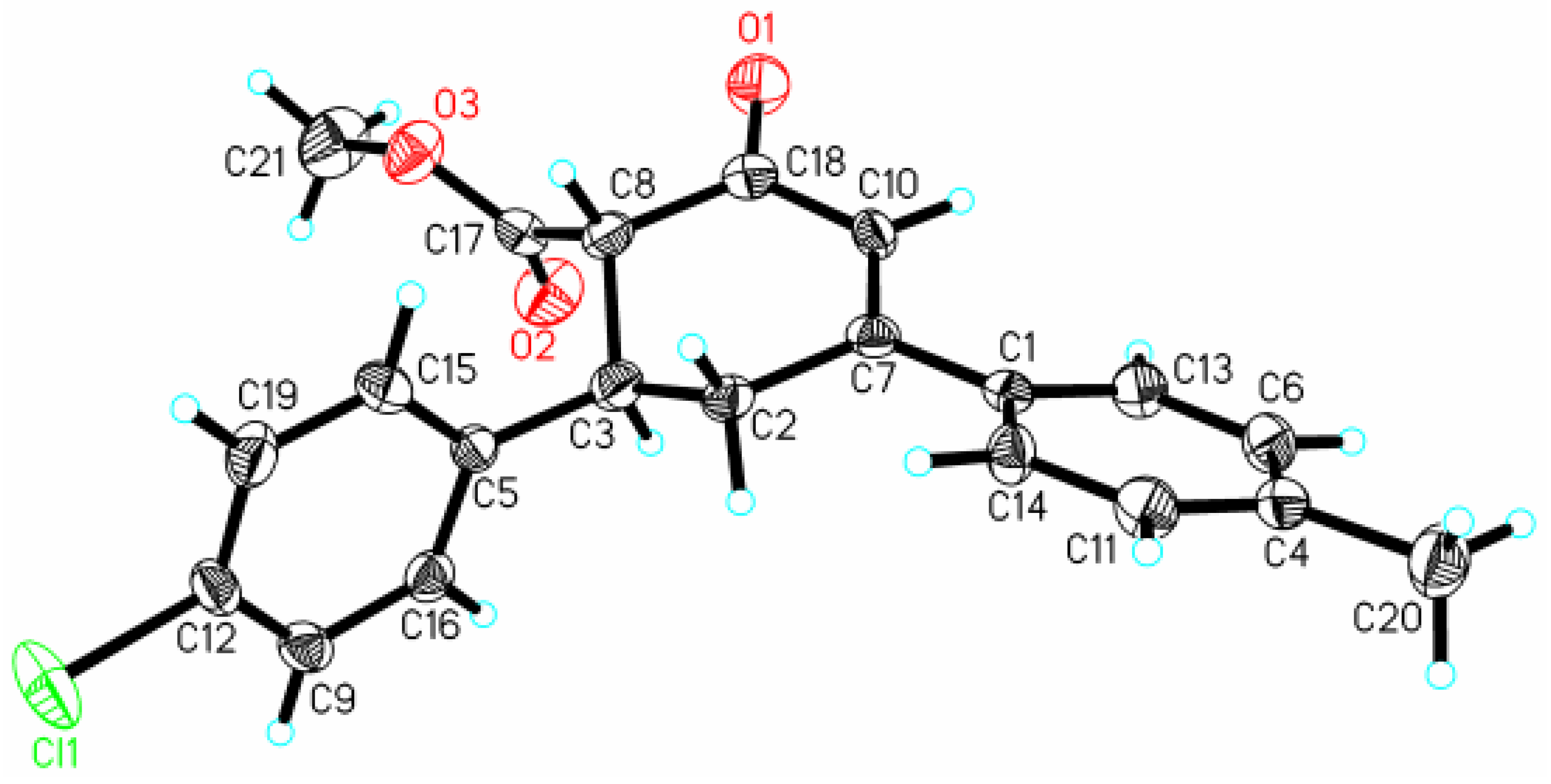
Structure of Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl) cyclohex-3-en-2-one-1-carboxylate
Introduction
Experimental
X-ray Crystallographic Data Collection and Structural Determination.
General procedure for the synthesis
Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl)cyclohex-3-en-2-one-1-carboxylate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Padmavathi, V.; Boggu, M.; Mohan, R.; Akula, B.; Katta, V.; Dandu, B. Synthesis of Some Fused Pyrazoles and Isoxazoles. Molecules 2000, 5, 1281–1286. [Google Scholar] [CrossRef] Balasankar, T.; Gopalakrishnan, M.; Nagarajan, S. Synthesis and antibacterial activity of some 5-(4-biphenylyl)-7-aryl[3,4-d][1,2,3]-benzothiadiazoles. Eur. J. Med Chem. 2005, 40, 728–731. [Google Scholar]
- Stanetty, P.; Kremslehner, M. Synthesis of thieno[3,2-d][1,2,3]thiadiazoles. New mechanistic aspects of the Hurd-Mori reaction. Heterocycles 1998, 48, 259–266. [Google Scholar] [CrossRef]
- Stanetty, P.; Kremslehner, M.; Müllner, M. Application of the Hurd-Mori reaction for the synthesis of tricyclic annelated 1,2,3-thiadiazoles. J. Heterocycl. Chem. 1996, 33, 1759–1763. [Google Scholar] [CrossRef]
- Lewis, G.; Nelson, P. 3-[(1,2,3-Thiadiazol-5-ylthio)methyl]cephalosporins. J. Med. Chem. 1979, 22, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, V.; Sharmila, K.; Padmaja, A.; Bhaskar, R. An efficient synthesis of 6,8-diarylcarbazoles via Fischer indole cyclizations. Heterocycl. Commun. 1999, 5, 451–456. [Google Scholar] [CrossRef]
- Jalbout, A.F.; Charris, L. unpublished results.

| Parameter | Compound 2 | Parameter | Compound 2 |
| formula | C21 H19 Cl O3 | ρ(calcd), g cm-3 | 1.308 |
| fw | 354.81 | F(000) | 744 |
| lattice | Monoclinic | μ(Mo KR), cm-1 | 2.28 |
| a, Å | 10.949(2) | diffractometer | APEX |
| b, Å | 14.047(3) | radiatn λ, Å | 0.71073 |
| c, Å | 12.343(2) | temp, ْ C | 25 |
| β, deg | 108.392(4) | R (I > 2.00σ(I))a | 0.1331 |
| V, Å3 | 1801.4(6) | Rw (all data)b | 0.2143 |
| space group | P2(1)/c | no. of observs | 3212 (all data) |
| Z | 4 | no. of variables | 228 |
| Distances | |
| Cl(1)-C(12) | 1.748(6) |
| O(1)-C(18) | 1.217(6) |
| O(2)-C(17) | 1.189(7) |
| O(3)-C(17) | 1.320(7) |
| O(3)-C(21) | 1.454(7) |
| C(1)-C(7) | 1.472(7) |
| C(2)-C(7) | 1.507(7) |
| C(2)-C(3) | 1.508(7) |
| C(3)-C(5) | 1.503(7) |
| C(3)-C(8) | 1.532(7) |
| C(4)-C(20) | 1.509(8) |
| C(7)-C(10) | 1.337(7) |
| C(8)-C(17) | 1.512(8) |
| C(8)-C(18) | 1.520(8) |
| C(10)-C(18) | 1.462(8) |
| angles | |
| C(7)-C(2)-C(3) | 114.2(4) |
| C(2)-C(3)-C(8) | 111.0(5) |
| C(10)-C(7)-C(2) | 118.9(5) |
| C(18)-C(8)-C(3) | 112.1(5) |
| C(7)-C(10)-C(18) | 124.2(5) |
| C(10)-C(18)-C(8) | 118.0(5) |
| torsion angles | |
| C(7)-C(2)-C(3)-C(5) | -179.6(4) |
| C(7)-C(2)-C(3)-C(8) | -51.3(6) |
| C(2)-C(3)-C(5)-C(16) | -106.7(6) |
| C(8)-C(3)-C(5)-C(16) | 126.9(5) |
| C(2)-C(3)-C(5)-C(15) | 66.7(7) |
| C(8)-C(3)-C(5)-C(15) | -59.7(7) |
| C(14)-C(1)-C(7)-C(10) | 151.8(5) |
| C(13)-C(1)-C(7)-C(10) | -28.9(8) |
| C(14)-C(1)-C(7)-C(2) | -26.5(7) |
| C(13)-C(1)-C(7)-C(2) | 152.8(5) |
| C(3)-C(2)-C(7)-C(10) | 26.4(7) |
| C(3)-C(2)-C(7)-C(1) | -155.1(5) |
| C(5)-C(3)-C(8)-C(17) | -62.5(6) |
| C(2)-C(3)-C(8)-C(17) | 171.2(5) |
| C(5)-C(3)-C(8)-C(18) | 175.1(5) |
| C(2)-C(3)-C(8)-C(18) | 48.8(6) |
| C(1)-C(7)-C(10)-C(18) | -176.8(5) |
| C(2)-C(7)-C(10)-C(18) | 1.5(8) |
| C(21)-O(3)-C(17)-O(2) | -1.0(9) |
| C(21)-O(3)-C(17)-C(8) | 178.7(5) |
| C(18)-C(8)-C(17)-O(2) | 62.7(7) |
| C(3)-C(8)-C(17)-O(2) | -61.4(7) |
| C(18)-C(8)-C(17)-O(3) | -117.0(5) |
| C(3)-C(8)-C(17)-O(3) | 119.0(5) |
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jalbout, A.F.; Contreras-Torres, F.F.; Lobo, G.M.; Charris, J.E. Structure of Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl) cyclohex-3-en-2-one-1-carboxylate. Molbank 2007, 2007, M559. https://doi.org/10.3390/M559
Jalbout AF, Contreras-Torres FF, Lobo GM, Charris JE. Structure of Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl) cyclohex-3-en-2-one-1-carboxylate. Molbank. 2007; 2007(5):M559. https://doi.org/10.3390/M559
Chicago/Turabian StyleJalbout, Abraham F., Flavio F. Contreras-Torres, Gabriela M. Lobo, and Jaimi E. Charris. 2007. "Structure of Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl) cyclohex-3-en-2-one-1-carboxylate" Molbank 2007, no. 5: M559. https://doi.org/10.3390/M559
APA StyleJalbout, A. F., Contreras-Torres, F. F., Lobo, G. M., & Charris, J. E. (2007). Structure of Methyl (1S, 6S) 6-(4-chlorophenyl)-4-(4-methylphenyl) cyclohex-3-en-2-one-1-carboxylate. Molbank, 2007(5), M559. https://doi.org/10.3390/M559




