Abstract
A synthesis of [2-nitro-5-(10,15,20-triphenylporphyrin-5-yl)-phenyl]phenyl-acetonitrile zinc complex by the reaction of the respective nitroporphyrin with the anion of benzyl cyanide, followed by addition of DDQ, is described. This is the first example of this type of process in the nitrophenyl side ring of meso-tetraarylporphyrin derivatives.
The synthesis of unsymmetrical, double functionalized in meso-aryl ring, porphyrins is of significant importance due to their potential application as sensitizers in photodynamic cancer therapy (PDT).1
Amongst other methods, a very attractive one seems to be oxidative nucleophilic substitution of hydrogen (ONSH2) in nitrophenyl moiety of the respective starting porphyrin. Herein, we present the first example of this type of functionalization in the reaction of 5-(4-nitrophenyl)-10,15,20-triphenylporphyrin zinc complex3 with phenylacetonitrile carbanion (in DMF, at room temperature) followed by addition of DDQ. This leads to the substitution of hydrogen product, in the position ortho- to the NO2 group, in 35% yield.
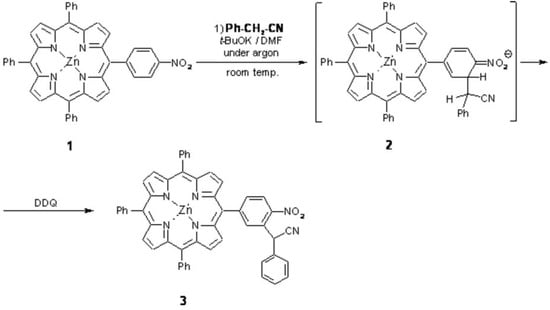
Scheme 1.
To a stirred solution of t-BuOK (71 mg, 0.63 mmol) in anhydrous DMF (5 mL, under argon), a solution of 5-(4-nitrophenyl)-10,15,20-triphenylporphyrin zinc(II)3 (1; 50 mg, 0.069 mmol) and benzyl cyanide (19 mg, 0.16 mmol) in DMF (3 mL) was added dropwise via syringe at room temperature during ca 2 min. After an additional 2 min of stirring, DDQ (23 mg, 0.10 mmol) was added in a one portion and the mixture was stirred for 3 min. Then, the mixture was poured into 3% HCl containing ice (80 mL). The precipitate was filtered, washed with water (3 × 15 mL), and then dissolved in CHCl3 (40 mL). After drying with anhydrous MgSO4 and evaporation of the solvent, the residue was chromatographed (silica gel 230-400 mesh; eluent: CHCl3 / n-hexane, 2: 1) to give: starting porphyrin 1 – 15.4 mg (31%), and product 3 – 13.9 mg (24%; 35% for recovered substrate).
Melting point: > 300 °C
IR (CH2Cl2, cm-1): 2246 (CN), 1598, 1583, 1525 (asym. NO2), 1487, 1441, 1340 (sym. NO2).
1H NMR (CDCl3, 200 MHz): 9.04 (d, J = 4.8 Hz, 1 H, Hβ-pyrrole), 8.99-8.94 (m, 5 H, Hβ-pyrrole), 8.89 (d, J = 4.8 Hz, 1 H, Hβ-pyrrole), 8.69 (d, J = 4.8 Hz, 1 H, Hβ-pyrrole), 8.65 (d, J = 1.6 Hz, 1 H, H-2 of H-Ar(NO2)), 8.49 (part of AB system, J = 8.3 Hz, 1 H, H-5 of H-Ar(NO2)), 8.40 (part of AB system coupled with another proton, J = 8.3 Hz, 1.6 Hz, 1 H, H-6 of H-Ar(NO2)), 8.28-8.16 (m, 6 H, H-Ph), 7.86-7.68 (m, 9 H, H-Ph), 7.58-7.31 (m, 5 H, CH(CN)Ph), 6.54 (s, 1 H, CH(CN)).
UV-VIS (CHCl3): λmax (log ε): 589.5 (3.79), 548.0 (4.47), 510.5 (3.70), 418.5 (5.55, Soret band), 348.0 nm (4.20).
MS (EI), m/z (% rel. int.): 836 (1.0, M+˙), 810 (0.9, M-CN), 733 (0.5), 678 (2), 677 (4), 676 (3), 675 (4), 674 (1.1), 77 (41), 44 (100, CO2+).
MS (ESI), m/z (% rel. int.): 842 (11), 841 (16), 840 (46), 839 (50), 838 (80), 837 (57), 836 (100) [isotopic M+]. The molecular formula was confirmed by comparing the theoretical and experimental isotope patterns for the M+ ion (C52H32N6O2Zn) – found to be identical within the experimental error limits.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Hsi, R.A.; Rosenthal, D.I.; Glatstein, E. Drugs 1999, 57, 725–734. Nyman, E.S.; Hynninen, P.H. J. Photochem. Photobiol. B 2004, 73, 1–28. [CrossRef]
- Mąkosza, M.; Staliński, K. Tetrahedron 1998, 54, 8797–8810. Mąkosza, M.; Staliński, K. Polish J. Chem. 1999, 73, 151–161.
- Ostrowski, S.; Mikus, A.; Shim, Y.K.; Lee, J.-Ch.; Seo, E.-Y.; Lee, K.-I.; Olejnik, M. Heterocycles 2002, 57, 1615–1626.
© 2006 MDPI. All rights reserved.