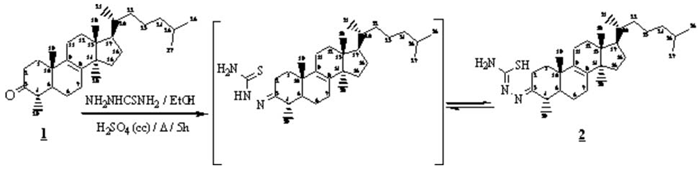
The compound 2 was prepared from equimolecular quantity of 1 (1g, 2.43 mmol), derivative triterpene resulting from Euphorbia officinarum1, and thiosemicarbazide2,3 dissolved in ethanol with several drops of conc. H2SO4. The mixture was heated at reflux for 5h, and evaporated under reduced pressure. The residue was purified on silica gel column using hexane: ethyl acetate (90:10) as eluent yielded compound 2 (0.99g, 2.06mmol) in 85% yield.
Melting point: 212-213 °C (Hexane)
MS (EI, 70eV): 485 (M+.)
1H NMR (300 MHz, CDCl3) d(ppm): 6.33 (NH); 7.25 (NH2); 8.74 (SH); 0.70 (3H-18, s); 0.96 (3H-19, s); 0.88 (3H-21, d, J = 6 Hz); 0.85 (3H-26, d, J = 2 Hz ); 0.86 (3H-27, d, J = 2 Hz ); 0.87 (3H-28, s); 1.20 (3H-29, d, J = 6 Hz ).
13C NMR (75 MHz, CDCl3) d (ppm): 36.50 (C-1); 37.35 (C-2); 159.35 (C-3) ; 50.58 (C-4); 49.98 (C-5); 21.75 (C-6); 28.12 (C-7); 132.35 (C-8); 135.73 (C-9); 36.36 (C-10); 21.55 (C-11); 25.45 (C-12); 44.54 (C-13); 49.75 (C-14); 30.86 (C-15); 29.81 (C-16); 46.10 (C-17); 15.82 (C-18); 18.15 (C-19); 36.14 (C-20); 18.65 (C-21); 36.56 (C-22); 24.35 (C-23); 32.41 (C-24); 34.58 (C-25); 21.72 (C-26); 21.92 (C-27); 24.22 (C-28); 12.25 (C-29); 106.12 (C-30); 179.18 (C=S).
MS (m/z): 485 (20%), 395 (42%), 282 (65%).
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
We would like to thank Pr. A. Idmessaaoud for helpful discussions.
References
- Benharref, A.; Lavergne, J.-P. Bull. Soc. Chim. Fr. 1985, 965–972.
- Beatriz, N.B.; et al. Arkivok. 2002, 14–23.
- Ourhriss, N.; Giorgi, M.; Mazoir, N.; Benharref, A. Acta Cryst. 2005, C61, o699–o701.
© 2006 MDPI. All rights reserved.