The products of aza-type Michael addition, i.e., β-amino carbonyl compounds and their derivatives, are often used as peptide analogs or precursors of optically active amino acids, amino alcohols, diamines, and lactams [1]. Moreover, β-amino carbonyl functionalities are ubiquitous motifs in natural products such as alkaloids and polyketides [2]. Herein, we report the synthesis of new product using aza-type Michael reactions under mild conditions.
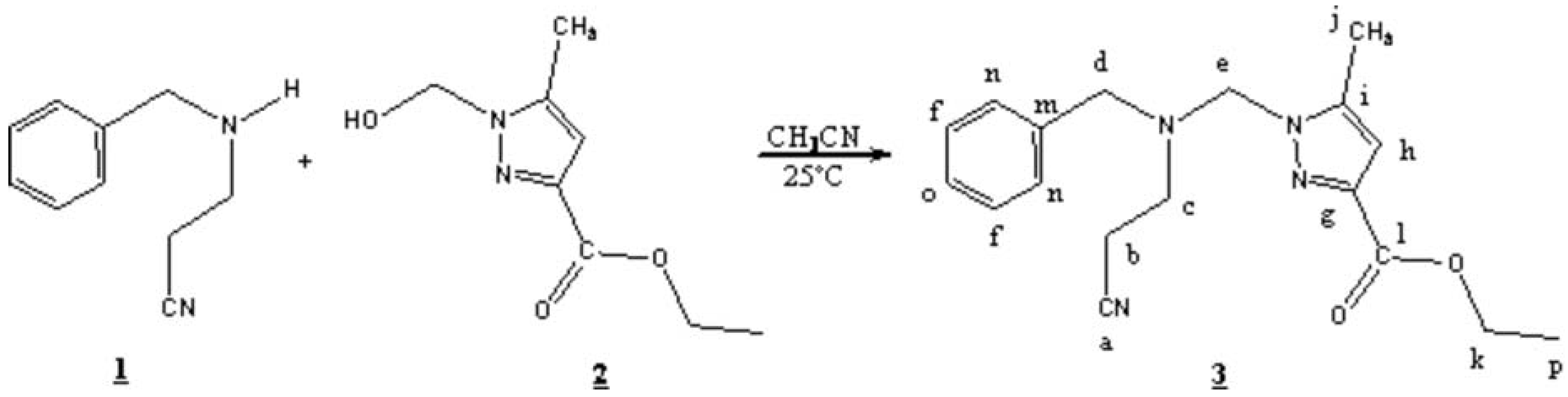
A mixture of 3-(benzylamino)propionitrile 1 [3] (1g, 6.25mmol) and 1-hydroxymethyl-5-methyl-1H-pyrazole-3-carboxylic acid ethyl ester 2 [4] (1.15g , 6.25 mmol) in 20 ml of acetonitrile was stirred at room temperature for four days, then the mixture was dried with Na2SO4 and filtered. The solvent was evaporated under reduced pressure. The product 3 obtained with an 81% yield as yellow oil.
1H-NMR (300 MHz, CDCl3): δ= 7.34 (CHarom, 5H, s) ; 6.55 ( CHpyr, 1H, s) ; 4.94 (pyr-CH2-N, 2H, s) ; 4.33-4.4 ( OCH2 , 2H, q, J = 8Hz) ; 3.750 (C6H5-CH2, 2H, s) ; 3.00-3.1 (CH2-CH2-CN, 2H, t, J = 7.33Hz) ; 2.34-2.38 ( N-CH2, 2H, t, J = 8Hz); 2.18 (CH3, 3H, s) and 1.39-1.34 ( CH3, 3H, t, J = 8Hz).
13C-NMR (CDCl3, 75 MHz): δ= 162.94 (l); 143.26 (i); 141.432 (g); 137.51 (m); 129.16; 129.06 (n); 128.83 (f); 128.23 (o); 119.00 (a); 109.19 (h); 67.70 (e); 61.26 (k); 56.76 (d); 48.34 (c); 17.44 (b); 14.77 (p); 11.55 (j).
EI-MS (70 eV, m/z): 173; 171; 154 (51.4); 119; 91 (60.6).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Fustero, S.; Pina, B.; Salavert, E.; Navarro, A.; Ramirez de Arellano, M. C.; Fuentes, A. S. J. Org. Chem. 2002, 67, 4667. [PubMed]
- Arend, M.; Westermann, B.; Risch, N. Angew. Chem., Int. Ed 1998, 37, 1045.
- Xu, L.W.; Li, L.; Xia, C.-G. Helvetica Chimica Acta 2004, 87, 1522.Azizi, N.; Saidi, M. R. Tetrahedron 2004, 60, 383.
- Touzani, R.; Ramdani, A.; El Kadiri, S.; Gourand, F. Molecules 1999, 4, M116.
© 2006 MDPI. All rights reserved.