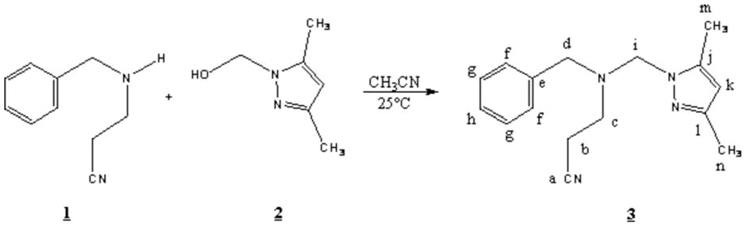
3-[Benzyl-(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Fustero, S.; Pina, B.; Salavert, E.; Navarro, A.; Ramirez de Arellano, M. C.; Fuentes, A. S. J. Org. Chem. 2002, 67, 4667. [PubMed]
- Arend, M.; Westermann, B.; Risch, N. Angew. Chem., Int. Ed 1998, 37, 1045.
- Xu, L.W.; Li, L.; Xia, C.-G. Helvetica Chimica Acta 2004, 87, 1522. [PubMed]Azizi, N.; Saidi, M. R. Tetrahedron 2004, 60, 383.
- Dvoretzky, I.; Richte, G. H. J. Org. Chem. 1950, 15, 1285.
© 2006 MDPI. All rights reserved.
Share and Cite
Herrag, L.; Touzani, R.; Ramdani, A.; Hammouti, B. 3-[Benzyl-(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile. Molbank 2006, 2006, M495. https://doi.org/10.3390/M495
Herrag L, Touzani R, Ramdani A, Hammouti B. 3-[Benzyl-(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile. Molbank. 2006; 2006(5):M495. https://doi.org/10.3390/M495
Chicago/Turabian StyleHerrag, Leila, Rachid Touzani, Abdelkrim Ramdani, and Belkheir Hammouti. 2006. "3-[Benzyl-(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile" Molbank 2006, no. 5: M495. https://doi.org/10.3390/M495
APA StyleHerrag, L., Touzani, R., Ramdani, A., & Hammouti, B. (2006). 3-[Benzyl-(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile. Molbank, 2006(5), M495. https://doi.org/10.3390/M495



