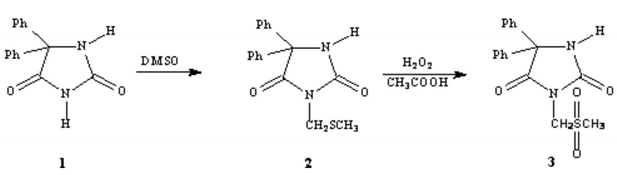
Synthesis of 5,5-diphenyl-3-(methylthiomethyl)hydantoin and 5,5-diphenyl-3-(methysulfonylomethyl)hydantoin
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Foye, W.O. Principles of Medicinal Chemistry; Lea & Febiger: Philadelphia, 1989. [Google Scholar]
- Avendano-Lopez, C.; Gonzalez-Trigo, G. Advances in Heterocyclic Chemistry; Katrizky, A.R., Ed.; Academic Press, Inc.: Orlando, 1985; Volume 38, chapter 4. [Google Scholar]
- Hudkins, R.L.; DeHaven-Hudkind, D.L. Bioorg. Med. Chem. Lett. 1994, 4, 2185.
- Czuba, W.; Grzegożek, M.; Kaniewska, A.; Nowak, K.; Poradowska, H.; Sędzik-Hibner, D.; Szpakiewicz, B.; Walkowicz, C. Pol. J. Chem. 1986, 60, 459.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.
Share and Cite
Grzegożek, M.; Nowak, K.; Szpakiewicz, B. Synthesis of 5,5-diphenyl-3-(methylthiomethyl)hydantoin and 5,5-diphenyl-3-(methysulfonylomethyl)hydantoin. Molbank 2006, 2006, M480. https://doi.org/10.3390/M480
Grzegożek M, Nowak K, Szpakiewicz B. Synthesis of 5,5-diphenyl-3-(methylthiomethyl)hydantoin and 5,5-diphenyl-3-(methysulfonylomethyl)hydantoin. Molbank. 2006; 2006(3):M480. https://doi.org/10.3390/M480
Chicago/Turabian StyleGrzegożek, Maria, Krystyna Nowak, and Barbara Szpakiewicz. 2006. "Synthesis of 5,5-diphenyl-3-(methylthiomethyl)hydantoin and 5,5-diphenyl-3-(methysulfonylomethyl)hydantoin" Molbank 2006, no. 3: M480. https://doi.org/10.3390/M480
APA StyleGrzegożek, M., Nowak, K., & Szpakiewicz, B. (2006). Synthesis of 5,5-diphenyl-3-(methylthiomethyl)hydantoin and 5,5-diphenyl-3-(methysulfonylomethyl)hydantoin. Molbank, 2006(3), M480. https://doi.org/10.3390/M480



