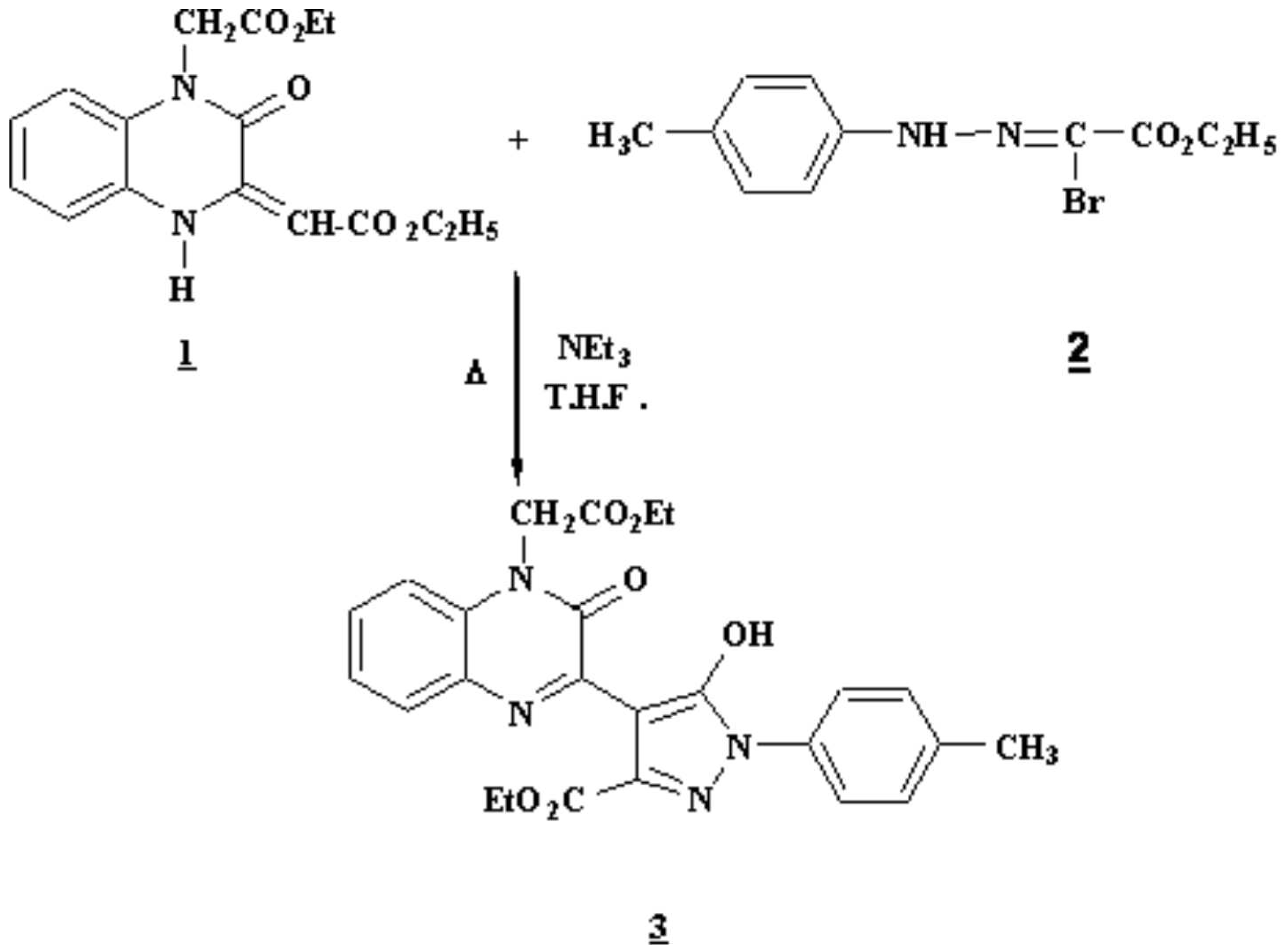
To a solution of hydrazonoyl bromide (5.70 g, 0.02 mol) 2 and 1 [1] (3.18 g, 0.01 mol) in tetrahydrofuran (60 ml) was added triethylamine (2.02 g, 0.02 mol in 10 ml of THF). The reaction mixture was refluxed for 48 hours. The solvent was evaporated under reduced pressure and the resulting crude material was chromatographied over silica gel column using 10:90 ethylacetate:hexane as eluent (yield 70%).
Melting point: 182-184°C
IR (KBr , cm−1 ): 1640 (nN-C=O); 1720 (nN-C=O).
1H- NMR ( 250 MHz, CDCl3): d= 7.81-7.04 (8H, m, H arom); 5.02 (2H, s, NCH2); 4.44 (2H, q, J=7 Hz, CH2); 4.25 (2H, q, J = 7 Hz, CH2); 2.36 (3H, s, CH3-Φ); 1.37 (3H, t, J = 7 Hz, CH3); 1.27 (3H, t, J = 7 Hz, CH3).
13C- NMR (250MHz, CDCl3): d= 166.5 (Cq); 164.2 (Cq); 161.6 (Cq); 154.5 (Cq); 143.8 (Cq); 143.4 (Cq); 135.9 (Cq); 135.5 (Cq); 129.4 (CHar); 129.1 CHar); 129.1 (Cq); 127.8 (CHar); 126.8 (Cq); 125.3 (CHar); 122.0 (CHar); 120.7 (CHar); 114.0 (CHar); 96 (C4); 62.3 (CH2); 61.7 (CH2); 44.2 (NCH2); 21.0 (Φ-CH3); 14.2 (CH3).
MS (IE): 476
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Ferfra, S.; Ahabchane, N.H.; Essassi, E. M. Molecules. submitted.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.