Semicarbazone as well as thiosemicarbazone of 5-acetyl-3-(methylsulfanyl)-1,2,4-triazine were synthesised as reactive intermediates for the synthesis of 1H-pyrazolo[4,3-e][1,2,4]triazine derivatives via acid promoted ring closure [1,2,3].
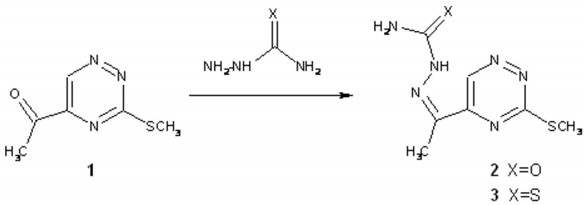
Starting ketone 1 was prepared according to a reported procedure [4]. To a solution of ketone 1 (169 mg, 1 mmol) and semicarbazone or thiosemicarbazone (1.2 mmol) in ethanol (20 ml) 10% HCl (0.5 ml) was added. The resulting reaction mixture was heated at reflux for 5 min and then stirred at room temperature for additional 30 min. The precipitated solid was filtered off, recrystallized from dioxane and dried under vacuum.
Semicarbazone of 5-acetyl-3-(methylsulfanyl)-1,2,4-triazine (2)
Yield 89%.
Melting Point: 260ºC.
1H-NMR (200 MHz, DMSO-d6): δ= 2.19 (s, 3H); 2.62 (s, 3H); 6.96 (s, 2H, NH2); 9.98 (s, 1H); 10.13 (s, 1H, NH).
IR (CHCl3 film, cm-1): 3339; 3106; 1693; 1520; 1486; 1426; 1312; 1256; 1183; 1133; 1058; 871; 713.
MS- EI (m/z, %): 226 (18) [M+]; 209 (7); 184 (11); 183 (100); 182 (62); 155 (12); 154 (39); 140 (32); 82 (49); 81 (30); 74 (18); 53 (19).
HR-MS: Calculated for C7H10N6OS: 226.0636. Found: 226.06314.
Thiosemicarbazone of 5-acetyl-3-(methylsulfanyl)-1,2,4-triazine (3)
Yield 87%.
Melting Point: 229ºC.
IR (CHCl3 film, cm-1): 3304; 3158; 2969; 1587; 1495; 1423; 1243; 1127; 1057; 876.
1H-NMR (200 MHz, DMSO-d6): δ= 2.31 (s, 3H); 2.63 (s, 3H); 8.53 (s, 1H); 8.70 (s, 1H); 10.08 (s, 1H); 10.81 (s, 1H).
MS-EI (m/z, %): 242 (80) [M+]; 199 (21); 167 (7); 154 (6); 141 (5); 140 (22); 128 (27); 116 (100); 82 (12); 81 (12); 74 (14); 60 (24); 53 (13).
HR-MS: Calculated for C7H10N6S2: 242.04084; Found: 242.04129.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.