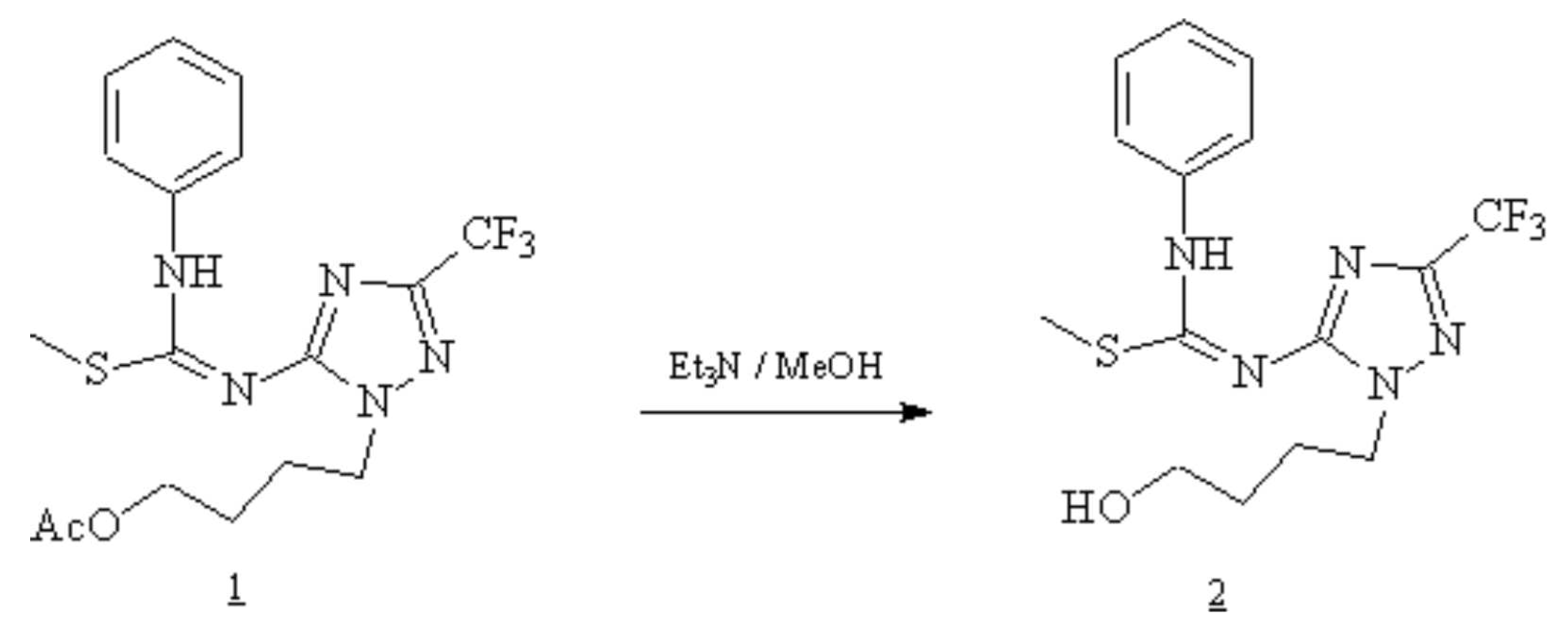
The desired compound 2 was obtained by complete deacetylation of compound 1 [1] using triethylamine [2]. To a solution of 1 (0.623g, 1.5 mmol) in methanol (15 ml) was added triethylamine (2 ml). The mixture was stirred at room temperature and the reaction was followed by TLC. After complete deacetylation (24 hours), the reaction mixture was evaporated and coevaporated with methanol (3x30 ml) under reduced pressure, then chromatographed over silica gel using CH2Cl2/MeOH (98:2 v/v) to give compound 2 as white powder crystallized from n-Hexane/ Ethylacetate.
Melting point: 113-114 °C.
Elemental Analysis: Calculated for C15H18F3N5SO: C, 48.26%; H, 4.83%; N, 18.77%. Found: C, 48.35%; H, 4.98%; N, 18.75%.
UV (EtOH, λmax): 277nm
IR (KBr, cm−1): 3448 (OH group)
MS (m/z): 374 (M+1)
1H-NMR (250 MHz, DMSO): δ= 1.7(m,2H,HOCH2CH2); 2.1(m,2H, CH2CH2-N); 2.1 (s,3H,SCH3); 3.7(t,2H,OCH2 ); 4.3 (m,2H,NCH2); 4.8(m,1H.OH, D2O exchangeable); 7.6-7.8 (m,5H, aromatic).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Haikal, A.; Zohdi, H. submitted to Molbank. 2003.
- Zohdi, H.; Haikal, a. Molecules 2001, 6, M263.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.