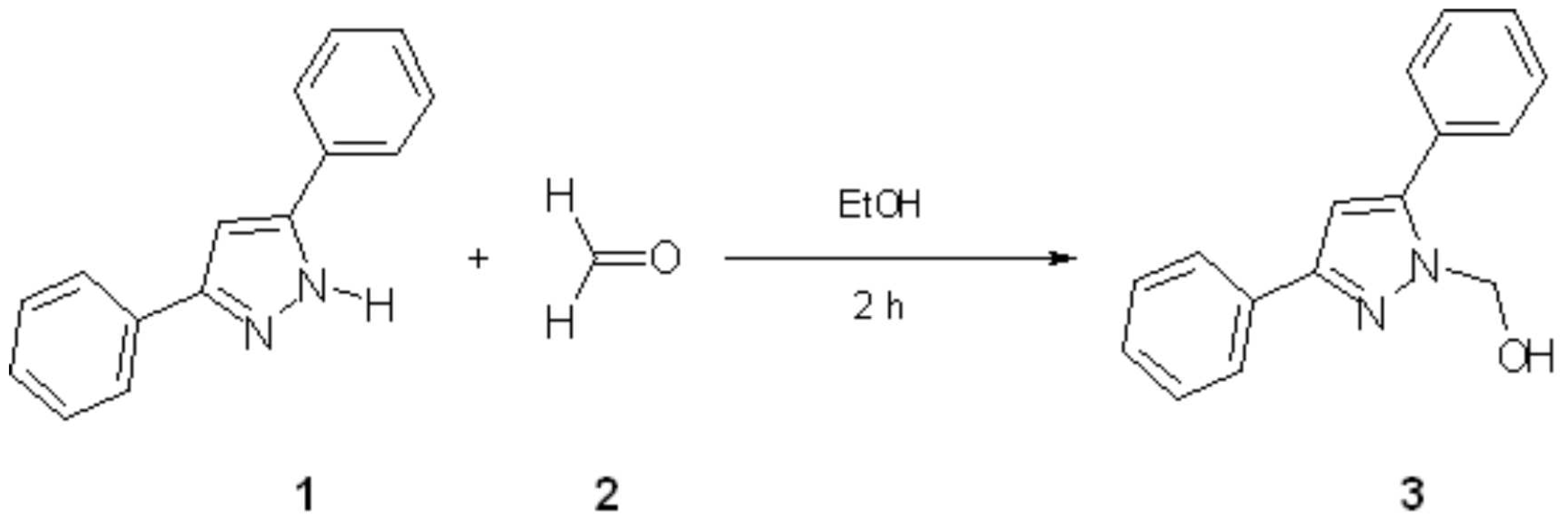
1-Hydroxymethyl-3,5-diphenylpyrazole
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes:
- Ali, S. S.; Ashraf, C. M.; Younas, M.; Ehsan, A. Pak. J. Sci. Ind. Res. 1993, 36, 12, 502-510.
- Driessen, W. J. R.. Neth. Chem. Soc. 1982, 101, 441.
- Bouabdallah, I.; Zidane, I; Ramdani, A. Rapport de DESA, Faculty of Sciences: Oujda, Maroc, 2001.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.
Share and Cite
Bouabdallah, I.; Ramdani, A.; Touzani, R. 1-Hydroxymethyl-3,5-diphenylpyrazole. Molbank 2005, 2005, M426. https://doi.org/10.3390/M426
Bouabdallah I, Ramdani A, Touzani R. 1-Hydroxymethyl-3,5-diphenylpyrazole. Molbank. 2005; 2005(3):M426. https://doi.org/10.3390/M426
Chicago/Turabian StyleBouabdallah, Ibrahim, Abdelkrim Ramdani, and Rachid Touzani. 2005. "1-Hydroxymethyl-3,5-diphenylpyrazole" Molbank 2005, no. 3: M426. https://doi.org/10.3390/M426
APA StyleBouabdallah, I., Ramdani, A., & Touzani, R. (2005). 1-Hydroxymethyl-3,5-diphenylpyrazole. Molbank, 2005(3), M426. https://doi.org/10.3390/M426



