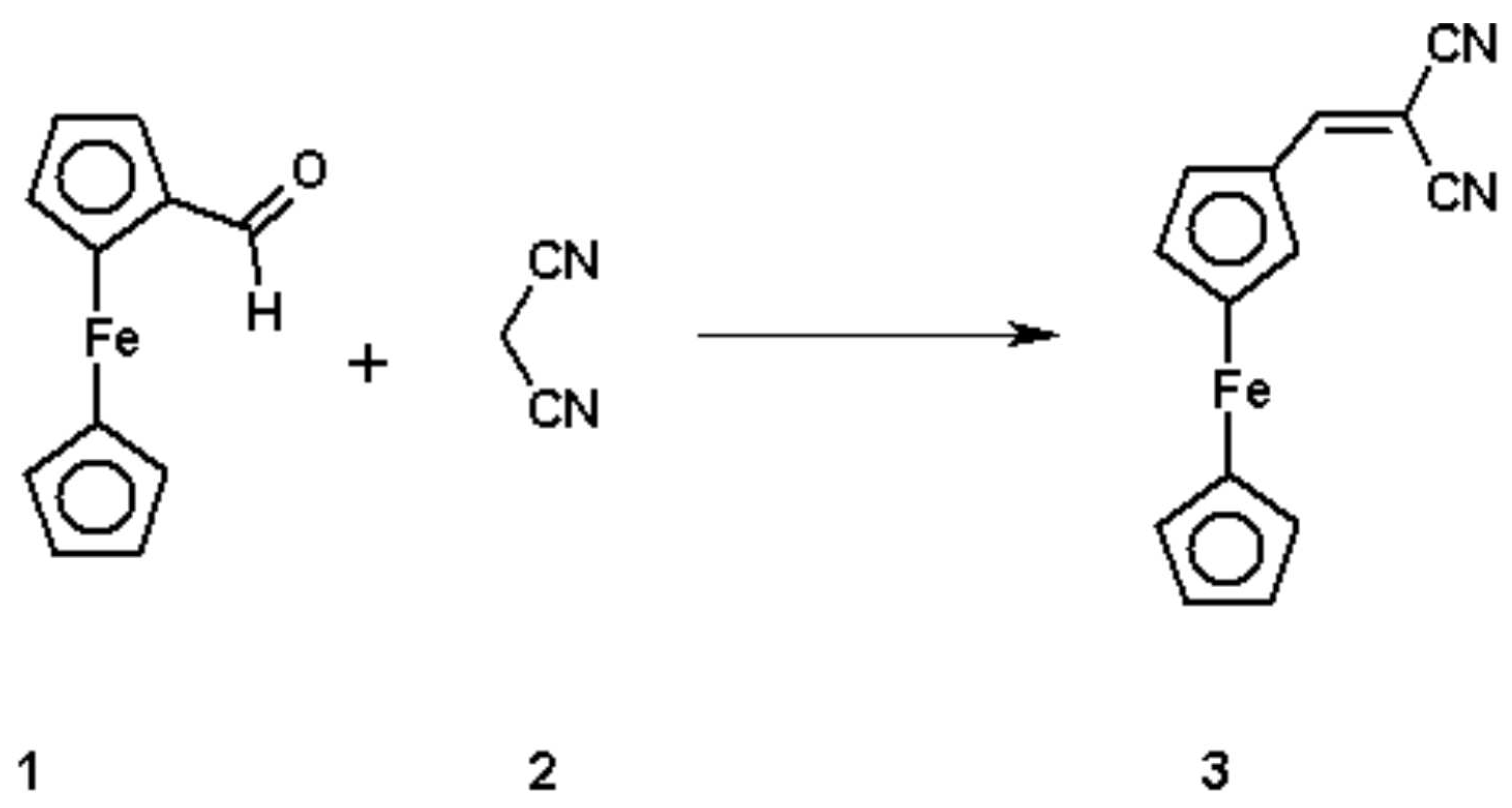
To a refluxed solution of ferrocenecarboxaldehyde 1 (2.14g, 0.01 mol) and malononitrile 2 (0.66g, 0.01 mmol) in ethanol (25 ml), Piperidine (1 ml) was added. After the addition, the solution became darker and the reflux was continued for six hours, then the solution was left to cool to room temperature and the products were precipitated. The precipitates were filter and washed with cold water and finally with ethanol, dried and recrystallized from ethanol to give the title compound 3. Deep red crystal (1.31g, 50%).
Melting point: 231-233 °C (uncorrected).
UV (EtOH; λmax nm; ε dm3.mol-1.cm-1): 345 (16376); 395 (4945); 521 (4913).
IR (KBr, cm-1): 2185, 2170 (CN); 1630 (C=C); 1101, 992, 814.
1H-NMR (400 MHz; CDCl3): δ= 7.70 (s, 1H, -CH=C); 5.01 (broad s, 2H, H-2, H4, H-5); 4.85 (broad s, 2H, H-2, H3, H-4); 4.33 (s, 5H, C5H5).
Elemental Analysis: Calculated for C14H10N2Fe (262.16): C 64.18%; H 3.82%; N 10.68%; Found; C 64.06%; H 4.01%; N 10.49%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Barlow, S.; Bunting, H. E.; Ringham, C.; Green, J. C.; Bublitz, G. U.; Boxer, S. G.; Perry, J. W.; Marder, S. R. J. Am. Chem. Soc. 1999, 121, 3715–3718.
- Asiri, A. M. Appl. Organometal. Chem. 2001, 15, 907–915.
© 2005 MDPI. All rights reserved.