Keywords:
1; 2; 4-triazine ketoxime; intramolecular cyclization; 1H-pyrazolo[4; 3-e][1; 2; 4]triazine As part of ongoing research programme on bicyclic heterocycles [1,2,3,4] we have elaborated a new approach to 1H-pyrazolo[4,3-e][1,2,4]triazine derivative 3 and its synthetic precursor 2 by reaction of oxime 5-acetyl-3-(methylsulfanyl)-1,2,4-triazine (1) with phenylhydrazine hydrochloride under different reaction conditions.
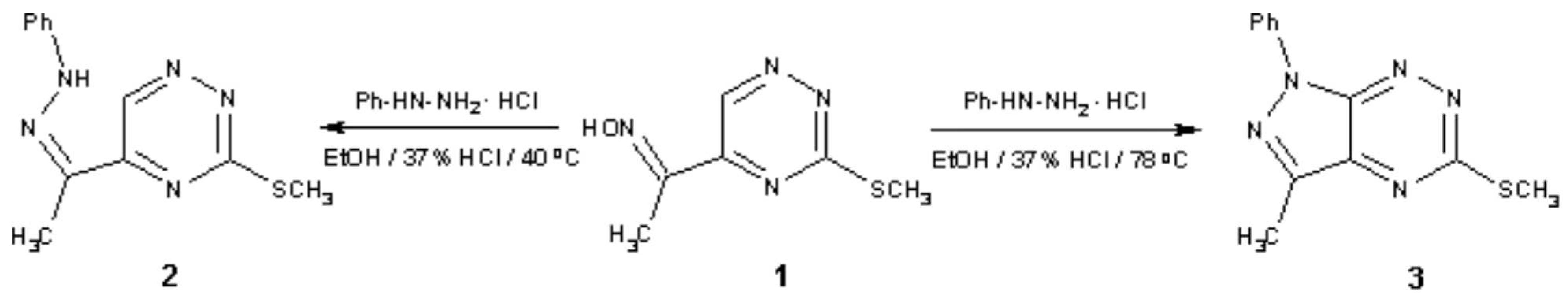
Phenylhydrazone of 5-acetyl-3-(methylsulfanyl)-1,2,4-triazine 2
To a solution of the oxime 1 (184 mg, 1 mmol) and phenylhydrazine hydrochloride (288 mg, 2 mmol) in ethanol (10 ml) 37% HCl (0.3 ml) was added. The mixture was heated at 40 oC for 9 hours and then the solvent was evaporated in vacuo. The solid was collected by filtration, washed with water and recrystallized from ethanol/water mixture (1:1) to give 2 in 27% yield.
Melting point: 224 oC.
1H-NMR (CDCl3): δ= 2.30 (s, 3H); 2.69 (s, 3H); 6.99-7.08 (m, 1H); 7.23-7.28 (m, 2H); 7.32-7.41 (m, 2H); 8.05 (s, 1H, NH); 9.63 (s, 1H).
IR (KBr, cm-1): 3240 (NH); 2980, 1600, 700.
EI-MS (70eV, m/z): 259 (7) [M+]; 147 (45); 129 (100); 112 (54); 70 (90).
Elemental Analysis: Calculated for C12H13N5S: C 55.60%; H 5.02%; N 27.03%. Found: C 55.53%; H 5.09%; N 26.99%.
3-Methyl –5-(methylsulfanyl)-1-phenyl-1H-pyrazolo[4,3-e][1,2,4]triazine 3
To a solution of the oxime 1 (184 mg, 1 mmol) and phenylhydrazine hydrochloride (216 mg, 1.5 mmol) in ethanol (10 ml) was added 37% HCl (0.3 ml). The mixture was heated at reflux for 5 hours and then the solvent was evaporated in vacuo. The solid was collected by filtration, washed with water and recrystallized from ethanol/water mixture (1:1) to give 3 in 18% yield.
Melting point: 105 oC.
1H-NMR (200 MHz, CDCl3): δ= 2.73 (s, 3H); 2.77 (s, 3H); 7.29-7.40 (m, 1H); 7.50-7.61 (m, 2H); 8.31-8.38 (m, 2H).
IR (KBr, cm-1): 2920, 1590, 1500, 1390, 760.
EI-MS (70eV, m/z): 257 (43) [M+]; 232 (3); 216 (22); 93 (41); 77 (100).
Elemental Analysis: Calculated for C12H11N5S: C 56.03%; H 4.28%; N 27.23%. Found: C 55.67%; H 4.13%; N 27.05%.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- Rykowski, A.; Mojzych, M.; Karczmarzyk, Z. Heterocycles 2000, 53, 2175.
- Karczmarzyk, Z.; Mojzych, M.; Rykowski, A. J. Chem. Cryst. 2000, 30, 423.
- Mojzych, M.; Rykowski, A. Polish J. Chem. 2003, 77, 1797.
- Mojzych, M.; Rykowski, A. Heterocycles 2004. (submited).
© 2005 MDPI. All rights reserved.