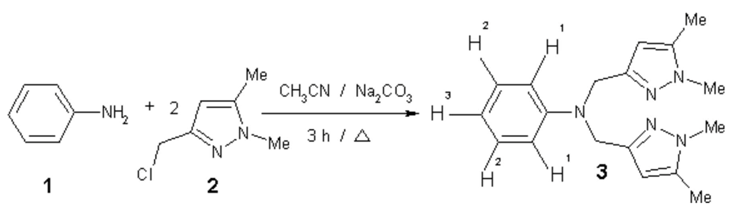
The mixture of aniline 1 (97 mg, 1 mmol), 3-chloromethyl-1,5-dimethyl pyrazol 2 (301.6 mg, 2 mmol) and sodium carbonate (444 mg, 4 mmol) in acetonitrile (10 mL) was refluxed for three hours [,]. The solvent was removed at reduced pressure. The residue was purified by recrystallysation to afford the produt 3 as a white solid. Yield: (205 mg, 69 %).
Melting point: 132-134°C (CH2Cl2).
IR (KBr, cm-1): 2930 (CH3); 1586 (C=C); 1500 (C=N); 1390, 1260, 1180, 1030, 990, 880.
1H-NMR (300 MHz, CDCl3) : δ= 7.14 (t, 2H, H2, J = 7.27 Hz) ; 6.87 (d, 2H, H1, J = 8.32 Hz) ; 6.64 (t, 1H, H3, J = 7.27 Hz); 5.86 (s, 2H, C-H pyrazol); 4.49 (s, 4H, N-CH2); 3.70 (s, 6H, N-CH3 ); 2.16 ( s, 6H, CH3).
13C-NMR (75 MHz, CDCl3): δ= 139.56; 129.32; 113.27; 104.50; 49.08; 36.25; 11.63.
EI-MS (70 eV, m/z): 309; 200; 109; 95; 77; 56.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes:
- Driessen, W. J. R. Neth. Chem. Soc. 1982, 101, 441.
- Malek, F. Thèse de Doctorat d’Etat; Faculté des Sciences: Oujda, Maroc, 1995. [Google Scholar]
- Sample Availability: Available from the Authors.
© 2005 MDPI. All rights reserved.