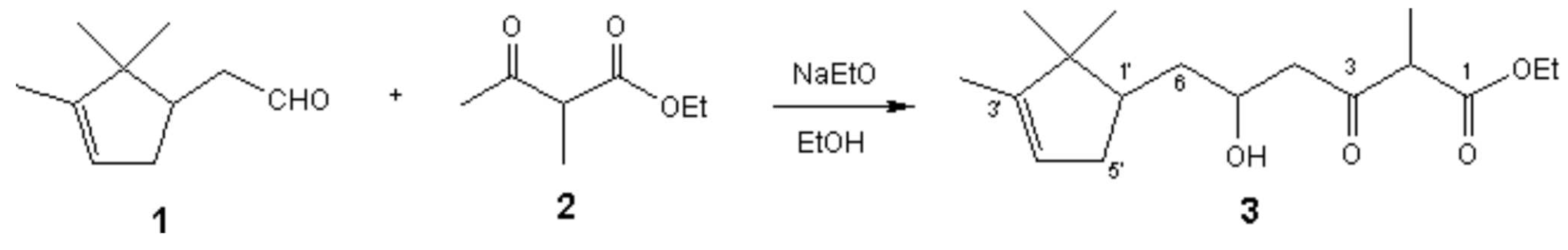
5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
© 2005 MDPI. All rights reserved.
Share and Cite
Pérez-Bonilla, M.; Castro, J.M.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. 5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester. Molbank 2005, 2005, M396. https://doi.org/10.3390/M396
Pérez-Bonilla M, Castro JM, Linares-Palomino PJ, Salido S, Altarejos J, Nogueras M, Sánchez A. 5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester. Molbank. 2005; 2005(1):M396. https://doi.org/10.3390/M396
Chicago/Turabian StylePérez-Bonilla, Mercedes, Juan M. Castro, Pablo J. Linares-Palomino, Sofía Salido, Joaquín Altarejos, Manuel Nogueras, and Adolfo Sánchez. 2005. "5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester" Molbank 2005, no. 1: M396. https://doi.org/10.3390/M396
APA StylePérez-Bonilla, M., Castro, J. M., Linares-Palomino, P. J., Salido, S., Altarejos, J., Nogueras, M., & Sánchez, A. (2005). 5-Hydroxy-2-methyl-3-oxo-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hexanoic acid ethyl ester. Molbank, 2005(1), M396. https://doi.org/10.3390/M396



