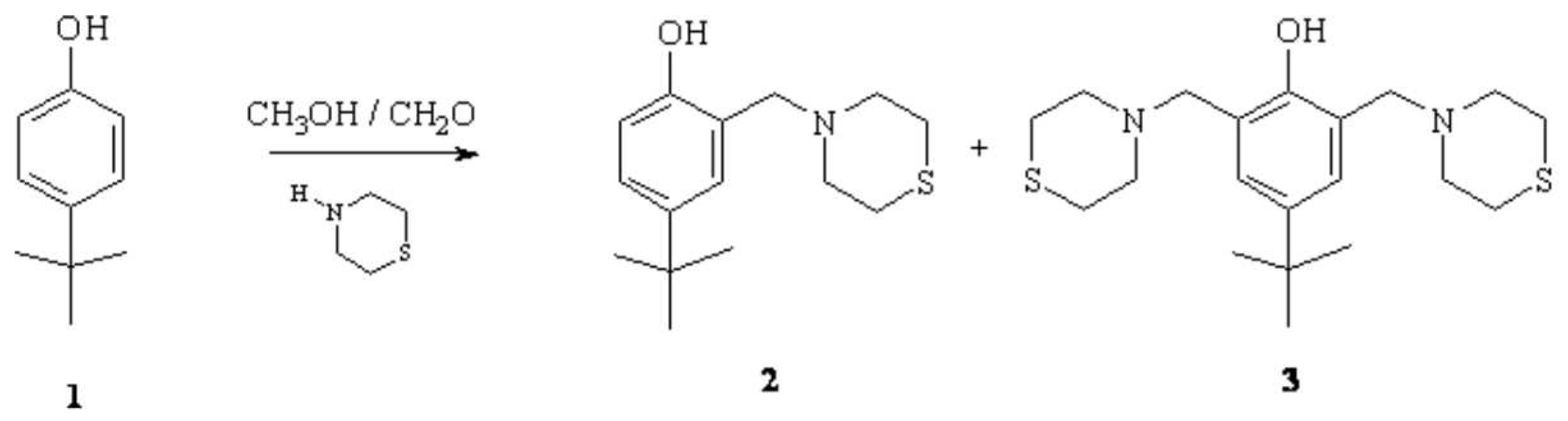
4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol (2) and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol (3) were prepared from 4-tert-butylphenol (1) and thiomorpholine and formaldehyde (37%) in methanol as solvent . A solution of methanol (50 mL) and 4-tert-butylphenol (1.49 g, 9.92 mmol) 1 was prepared and and heated at 40 °C for 15 minutes, after that a solution of thiomorpholine (2.0 g, 20.7mmol) and formaldehyde (1.50 mL, 20.15 mmol) in methanol were added. When the addition was completed, the reaction mixture was stirred at reflux for 24 hrs. The solvent was eliminated using rotavapor and reaction mixture was poured into water and extracted with ethyl acetate. Chromatography on silica gel (30/70 EtOAc/n-hexane) afforded two crystalline products 2 and 3 (5% and 35 % yield).
4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol (2)
Melting Point: 85-87 C° (methanol, uncorrected).
IR (CHCl3 film; cm-1): 3456 (O-H); 3197 (Csp2-H Ar); 2886 (Csp3-H).
1H-NMR (300 MHz; CDCl3): δ= 10.33 (1H, s, OH); 7.18 (1H, dd, J= 8.4Hz, 2.7Hz); 6.94 (1H, d, J= 2.7Hz); 6.74 (1H, d, 8.4Hz); 3.70 (2H, s, Ar-CH2); 2.82 (4H, m, -S-CH2-); 2.71 (4H, m, -N-CH2-); 1.27 (9H, CH3).
13C-NMR (75 MHz; CDCl3): δ= 155 (C); 141.8 (C); 125.60 (CH); 125.49 (CH); 119.77(C); 115.47 (CH); 62.51 (Ar-CH2); 54.36 (-N-CH2-); 33.84 (C); 31.48 (CH3); 27.79 (-S-CH2-).
MS (FAB; m/z, %): 266(80%); 265 (100%); 163(45%).
Elemental Analysis: Calculated for C15H23NOS: C, 67.88%; H, 8.73%; N, 5.28%; O, 6.03%; S, 12.08%. Found: C, 67.58%; H, 8.75%; N, 5.41%; O, 6.09%; S, 12.01%.
4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol (3)
Melting Point: 95-97 C° (methanol, uncorrected).
IR (CHCl3 film; cm-1): 3403 (O-H); 3089 (Csp2-H Ar); 2986 (Csp3-H).
1H-NMR (300 MHz; CDCl3): δ=10.69 (1H, s, OH); 7.09 (2H, s); 3.71 (4H, s, Ar-CH2); 2.86 (8H, m, -S-CH2-); 2.76 (8H, m, -N-CH2-); 1.27 (9H, CH3).
13C-NMR (75 MHz; CDCl3): δ=153.6 (C); 141.14 (C); 125.79 (CH); 121.22 (C); 58.81 (Ar-CH2); 54.42 (-N-CH2-); 33.78 (C); 31.47 (CH3); 27.74 (-S-CH2-).
MS (FAB; m/z, %): 381 (35%); 278 (100%); 175 (50%).
Elemental Analysis: Calculated for C20H32N2OS2: C, 63.11%; H, 8.47%; N, 7.36%; O, 4.20%; S, 16.85%. Found: C, 63.42%; H, 8.51%; N, 7.29%; O, 4.25%; S, 16.91%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgements
The authors wish to acknowledge to PAPIIT/UNAM Projects No IN205902 and IN207705 and ALPHARMA SA de CV, by partially support this work. We would like to thank C.Barajas, F.Sotres, P.García, D.Jiménez from FESC-UNAM and Rosa I.del Villar M., Oscar Yañez and Georgina Duarte from USAI-UNAM for their skillful technical assistance and DGSCA-UNAM for their support. As a part of Project Cátedra Química Medicinal of FESC-UNAM.
References
- Biava, M.; Fioravanti, R.; Porretta, G.C.; Deidda, D.; Maullu, C.; Pompei, M. Biorg.& Med. Chem. Lett. 1999, 9, 2983–2988.
- Teipel, S.; Griesar, K.; Haase, W.; Krebs, B. Inorganic Chemistry 1994, 33, 456–64.
- Hodgkin, J.H. Aust. J. Chem. 1984, 37, 2371–2378.
© 2005 MDPI. All rights reserved.