Synthesis of 4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol, and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol
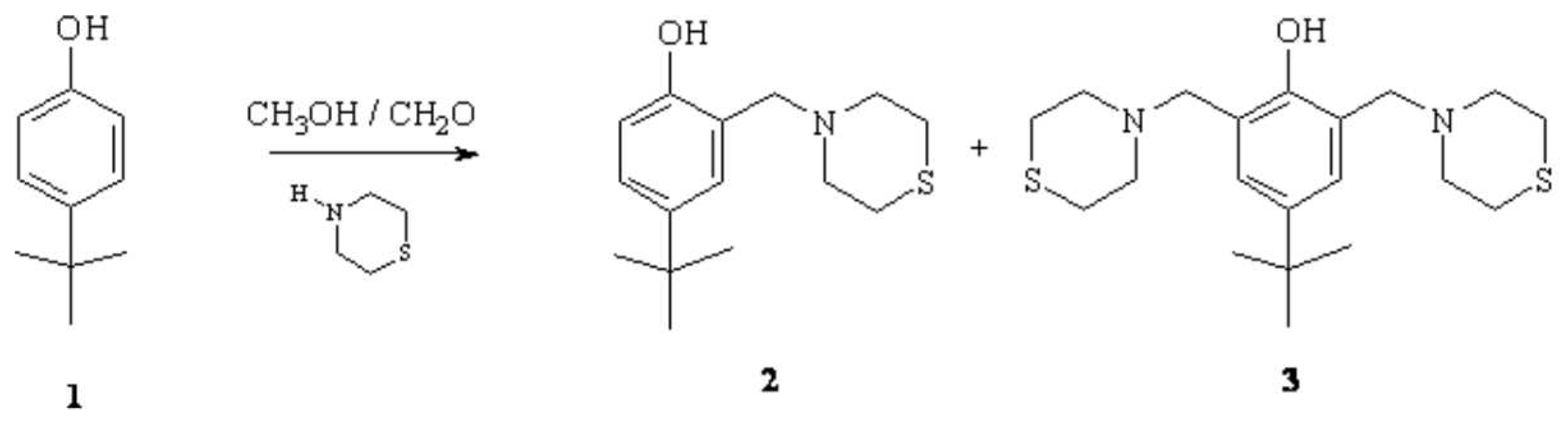
4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol (2)
4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgements
References
- Biava, M.; Fioravanti, R.; Porretta, G.C.; Deidda, D.; Maullu, C.; Pompei, M. Biorg.& Med. Chem. Lett. 1999, 9, 2983–2988.
- Teipel, S.; Griesar, K.; Haase, W.; Krebs, B. Inorganic Chemistry 1994, 33, 456–64.
- Hodgkin, J.H. Aust. J. Chem. 1984, 37, 2371–2378.
© 2005 MDPI. All rights reserved.
Share and Cite
Velázquez, A.M.; Torres, L.; Gonzále, R.; Martínez, I.; Valencia, A.; Pecin, A.; Torres, L.; Mencon, I.; Martínez, L.; Ramírez, A.; et al. Synthesis of 4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol, and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol. Molbank 2005, 2005, M401. https://doi.org/10.3390/M401
Velázquez AM, Torres L, Gonzále R, Martínez I, Valencia A, Pecin A, Torres L, Mencon I, Martínez L, Ramírez A, et al. Synthesis of 4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol, and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol. Molbank. 2005; 2005(1):M401. https://doi.org/10.3390/M401
Chicago/Turabian StyleVelázquez, A. Ma., L. Torres, R. Gonzále, I. Martínez, A. Valencia, A. Pecin, L. Torres, I. Mencon, L. Martínez, A. Ramírez, and et al. 2005. "Synthesis of 4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol, and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol" Molbank 2005, no. 1: M401. https://doi.org/10.3390/M401
APA StyleVelázquez, A. M., Torres, L., Gonzále, R., Martínez, I., Valencia, A., Pecin, A., Torres, L., Mencon, I., Martínez, L., Ramírez, A., Hernández, R., López-Castañares, R., Olvera-Neria, O., & Angeles, E. (2005). Synthesis of 4-tert-butyl-2-(thiomorpholin-4-ylmethyl)phenol, and 4-tert-butyl-2,6-bis(thiomorpholin-4-ylmethyl)phenol. Molbank, 2005(1), M401. https://doi.org/10.3390/M401