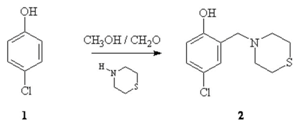
4-chloro-2-(thiomorpholin-4-ylmethyl)phenol (2) was prepared from 4-chlorophenol (1) and thiomorpholine and formaldehyde (37%) in methanol as solvent . A solution of methanol (50 mL) and 4-chlorophenol 1 (1.26g, 9.80 mmol) was prepared and heated at 40 °C for 15 minutes, after that a solution of thiomorfoline (2.0g, 20.7 mmol) and formaldehyde (1.5 mL, 20.15 mmol) in methanol was added . When the addition was completed, the reaction mixture was stirred at reflux for 24 hrs. The solvent was eliminated using rotavapor and reaction mixture was poured into water and extracted with ehtyl acetate. The product was crystallized after eliminated solvent and recrystallized from ethanol as white powder (2) (25% yield).
Melting Point: 127-129 °C (methanol, uncorrected).
IR (CHCl3 film, cm-1): 3502 (O-H); 3010 (Csp2-H Ar); 2985 (Csp3-H).
1H-NMR (300 MHz; CDCl3): δ= 10.56 (1H, s, OH); 7.11 (1H, dd, J= 8.7Hz, 2.7Hz); 6.94 (1H, d, J= 2.7Hz); 6.74 (1H, d, 8.7Hz); 3.65 (2H, s, Ar-CH2); 2.81 (4H, m, -S-CH2-); 2.71 (4H, m, -N-CH2-).
13C-NMR (75 MHz; CDCl3): δ= 156 (C); 128.56 (CH); 128.31 (CH); 123.61 (C); 122.11(C); 117.37 (CH); 61.63 (Ar-CH2); 54.27 (-S-CH2-); 27.73 (-N-CH2-).
MS (FAB; m/z, %): 244(100%); 215; 180; 154.
Elemental Analysis: Calculated for C11H14NOSCl: C, 54.20%; H, 5.79%; N, 5.75%; O, 6.56%; S, 13.15%; Cl, 14.54%. Found: C, 54.40%; H, 5.77%; N, 5.80%; O, 6.45%; S, 13.22%; Cl, 14.49%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors wish to acknowledge to PAPIIT/UNAM Projects No IN205902 and IN207705 and ALPHARMA SA de CV, by partially support this work. We would like to thank C.Barajas, F.Sotres, P.García, D.Jiménez from FESC-UNAM and Rosa I.del Villar M., Oscar Yañez and Georgina Duarte from USAI-UNAM for their skillful technical assistance and DGSCA-UNAM for their support. As a part of Project Cátedra Química Medicinal of FESC-UNAM.
References
- Biava, M.; Fioravanti, R.; Porretta, G.C.; Deidda, D.; Maullu, C.; Pompei, M. Biorg.& Med.Chem.Lett. 1999, 9, 2083–2985.
- Teipel, S.; Griesar, K.; Haase, W.; Krebs, B. Inorganic Chemistry 1994, 33, 456–64.
- Hodgkin, J.H. Aust .J. Chem. 1984, 37, 2371–2378.
© 2005 MDPI. All rights reserved.