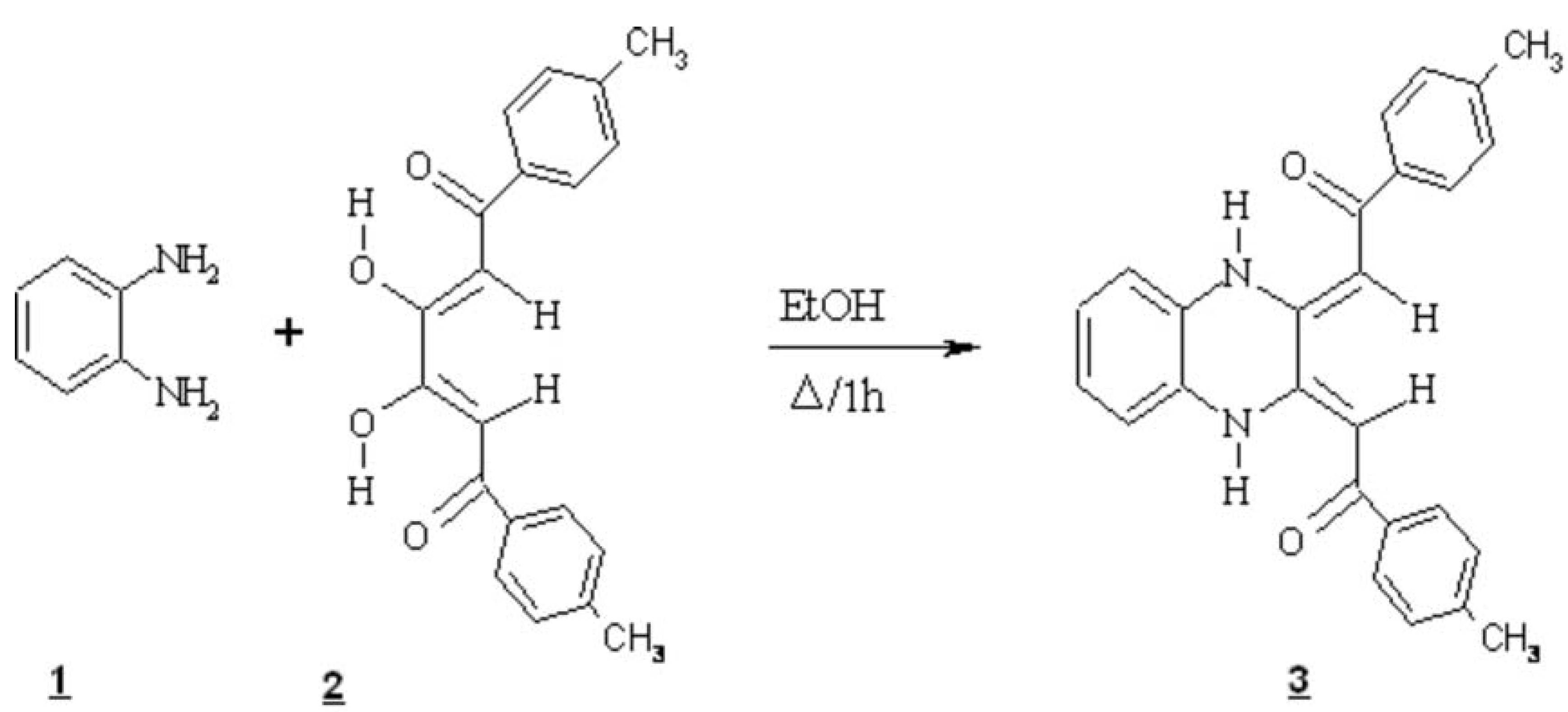
The product 3 was prepared by the condensation of dienone 2 [1] (1g, 3.11 mmol) with o-phenylenediamine 1 (0.33g, 3.11 mmol) by refluxing an ethanol (50 mL) solution for 1 h. under nitrogen [2,3]. The brown solid that formed, was collected by filtration and was purified by recrystallization in (acetone-chloroform : 50-50). The colour of the compound: red, yield : (1g, 81%).
Melting point : 198-200°C (acetone-chloroform)
IR (KBr, cm-1) : 1570 (C=O) ; 1410 (C=C).
1H-NMR (200 MHz, CDCl3) δ : 7.9 (d, 4H, J= 8.06Hz, -CH Benz) ; 7.31 (d, 4H, J= 8.06Hz, CH Benz) ; 7.15-7.17 (m, 4H, -CH Benz) ; 6.51 (s, 2H, -CHCO) ; 2.44 (s, 6H, 2CH3).
E. A: Anal. Calc for: C26H22N2O2 .C6H6.H2O , C : 78.34, H : 6.16, N : 5.71
Found , C : 79.16, H : 5.78, N : 5.86.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Saalfrank, R.W.; Lôw, N.; Demleitner, B.; Stalk, D.; Teichert, M. Chem. Eur. J. 1998, 4, 1305–1311.
- Touzani, R.; Ben-hadda, T.; El Kadiri, S.; Ramdani, A.; Maury, O.; Le Bozec, H.; Toupet, L.; Dixneuf, P.H. New J. Chem. 2001, 25, 391–395.
- Waring, M.J.; Ben-hadda, T.; Kotchevar, A.T.; Ramdani, A.; Touzani, R.; El Kadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. Molecules 2002, 7, 641–656.
- Benzene was used in a former cristallization
- Sample Availability: Available from the Authors.
© 2004 MDPI. All rights reserved.