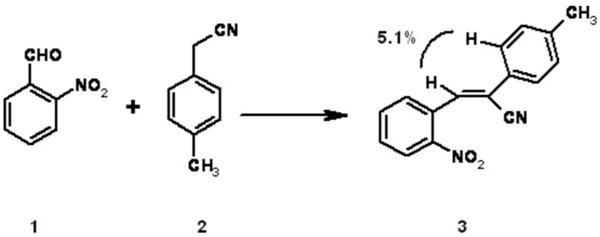
(2Z)-3-(2-Nitrophenyl)-2-(4-methylphenyl)acrylonitrile (3) was prepared by Knoevenagel condensation of 2-nitrobenzaldehyde 1 and 4-methylphenylacetonitrile 2 in ethanol using KOH as a base [1,2]. 2-Nitrobenzaldehyde 1 (3.01 g, 0.02 mol) and 4-methylphenylacetonitrile 2 (3.32 g, 0.02 mol ) in ethanol (35 mL) were heated under reflux for seven minutes. Potassium hydroxide (1.12 g, 0.02 mol) was added in one portion and the reflux was continued for two hours. The reaction mixture was cooled to room temperature and the solid formed was filtered, washed with water and finally with ethanol (2 x 30 mL) and dried. The product was recrystallized from ethanol as yellow crystals (2.63g, 95%).
M.p. 142-144 ºC.
UV lmax (nm; EtOH)/e (dm3.mol-1.cm-1) 350/15500.
IR (cm-1; KBr Disk) 2215 (CN), 1606 (C=C).
1H-NMR (400 MHz; CDCl3, Me4Si, dH): 8.24 (1H, d, J= 9.4 Hz), 8.00 (1H, s, CH=C ), 7.94 (1H, d, J = 7.7 Hz), 7.77 (1H, d), 7.64 ( 1H, dd), 7.60 (2H,d, 8.1 Hz), 7.28 ( 2H, d, 8.2 Hz), 2.38 (3H, s, MePh).
13C-NMR (100 MHz; CDCl3, Me4Si, dC): 21.2, 108.6, 110.5, 110.8, 118.8, 124.2, 125.6, 126.8, 129.7, 131.9, 138.9, 141.1, 148.9 and 150.9.
Elemental Analysis: Calculated for C16H12N2O2 (264.26): C 72.72, H 4.58, N 10.60; Found: C 72.61, H 4.76, N 10.45.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Jones, G. Org. React. 1967, 15, 204–599.
- Stewart, J. T.; Kim, M. J. Chem. Eng. Data 1987, 32, 387–389.
© 2004 MDPI. All rights reserved.