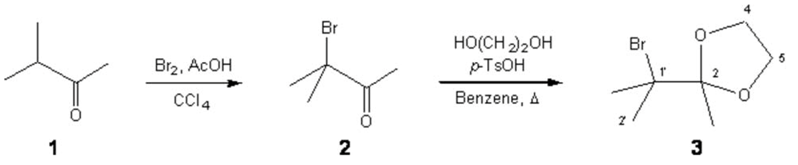
A solution of bromine (0.3 mL, 5.90 mmol) in CCl4 (2 mL) was slowly added over a stirred mixture of 3-methyl-butan-2-one (1) (505 mg, 5.90 mmol) and AcOH (0.33 mL) at room temperature. After complete addition of bromine it was left reacting for 1 h and then, the reaction was quenched by pouring carefully aqueous NaHSO3 (25 mL, 40% w/v). The organic layer was washed with 40% NaHSO3 (25 mL), saturated NaHCO3 (3×25 mL) and brine (3×25 mL). The organic layer was dried over anhydrous Na2SO4, and 15 mL of MeOH were added to evaporate the azeotrope under reduced pressure. The residue (1.03 g), which contains mainly 3-bromo-3-methyl-butan-2-one (2), was resolved in benzene (20 mL) and anhydrous p-TsOH (171 mg, 1.00 mmol) and ethyleneglycol (928 mg, 14.90 mmol) were added. Then a Dean-Stark trap device was fit and the reaction refluxed for 4.5 h. The crude reaction was worked up by washing with saturated NaHCO3 (3×25 mL) and brine (4×25 mL) and the organic layer dried over anhydrous Na2SO4, after that, MeOH (15 mL) was added to evaporate the azeotrope under reduced pressure. The residue (811 mg) was purified by reduced pressure distillation (0.15 mmHg, 32 ºC) to yield the title compound 3 (770 mg, 3.70 mmol, 62% from 1) as a colorless liquid.
IR (neat, n, cm−1): 1161, 1093, 1045, 951 (C-O-C), 649 (C-Br).
1H NMR (300 MHz, CDCl3, d, ppm): 1.52 (3H, s, Me-2), 1.79 (6H, s, 2Me-1’), 4.05 (4H, br s, H-4, H-5).
13C NMR (75 MHz, CDCl3, d, ppm): 20.52 (Me-2), 29.51 (C-2’, Me-1’), 65.93 (C-4, C-5), 69.99 (C-1’), 111.92 (C-2).
MS (70 eV, m/z): 195 ([M+2]+–Me, 1%), 193 (M+–Me, 2), 129 (M+–Br, 5), 153 (C3H681Br+, 1), 121(C3H679Br+, 1), 114 (M+–Br–Me, 6), 99 (M–Br–2Me, 5), 87 (C4H7O2+, 98), 69 (9), 57 (22), 43 (100).
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements:
We wish to thank the Ministerio de Ciencia y Tecnología for financial support (R+D Project PPQ2000-1665) and the Ministerio de Educación, Cultura y Deporte for a fellowship to J. M. Castro.
© 2004 MDPI. All rights reserved.