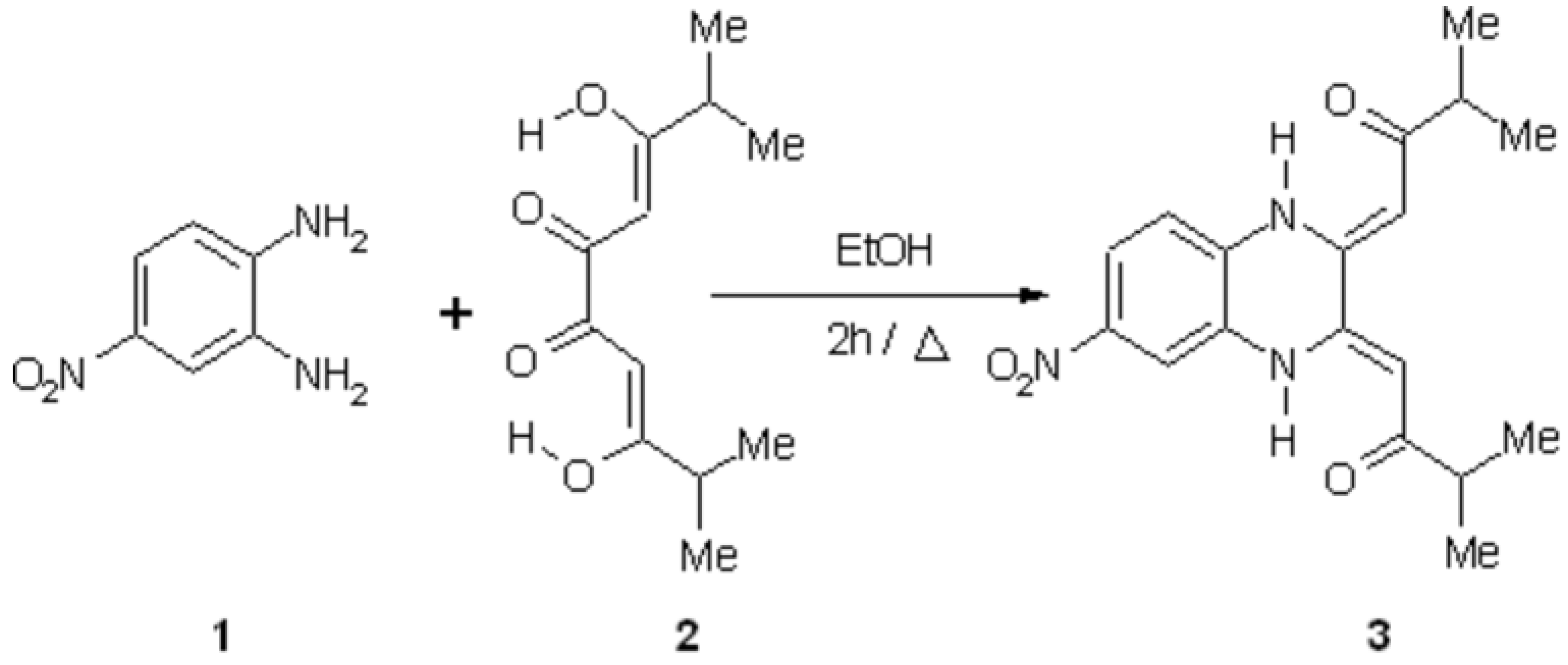
To a solution of 3,8-dihydroxy-2,9-dimethyl deca-3,7-diene-5,6-dione 2 [1] (1.278 g, 5.6 mmol) in absolute ethanol (20 mL) was added the 4-nitro-orthophenylenediamine 1 (0.865 g, 5.6 mmol) and the mixture was refluxed for 2 hours [2]. The yellow precipitate 3 was filtered off, washed with ethanol and dried in air.
Yield: (780 mg , 41 %).
Melting point: 150-152 °C (EtOH).
IR (KBr, cm-1): 2920 (CH of CH3) ; 1610 (C=O).
1H-NMR (200 MHz, CDCl3, d, ppm): 14.26 (s, 2H, N-H); 14.21 (s, 2H, N-H); 7.95 (d, 1H, C-H Benz, J = 8.4 Hz); 7.91 (s, 1H, C-H Benz); 7.05 (d, 1H, C-H Benz, J = 8.4 Hz); 5.86 (s, 1H, =CH); 5.82 (s, 1H, =CH); 2.72 (m, 2H, CH(CH3)2, J = 6.9 Hz); 1.21 (d, 12 H, CH3, J = 6.9 Hz).
HR/MS (C18H21N3O4): calc 343.1541; found 343.1532.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and notes:
- Bouabdallah, I.; Zidane, I.; Malek, F.; Touzani, R.; El Kodadi, M.; Ramdani, A. Molbank 2003, M 345. [PubMed]
- Waring, M.; Ben-hadda, T.; Kotchevar, A.; Ramdani, A.; Touzani, R.; El Kadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. Molecules 2002, 7, 641–656.
- Sample Availability: Available from the Authors.
© 2004 MDPI. All rights reserved.