As part of a research programme targeting novel molecules derived from o-hydroxyaryl ketone hydrazones[1,2], we synthesised 2,2´-dihydroxybenzophenone N-carbonylethoxyhydrazone.
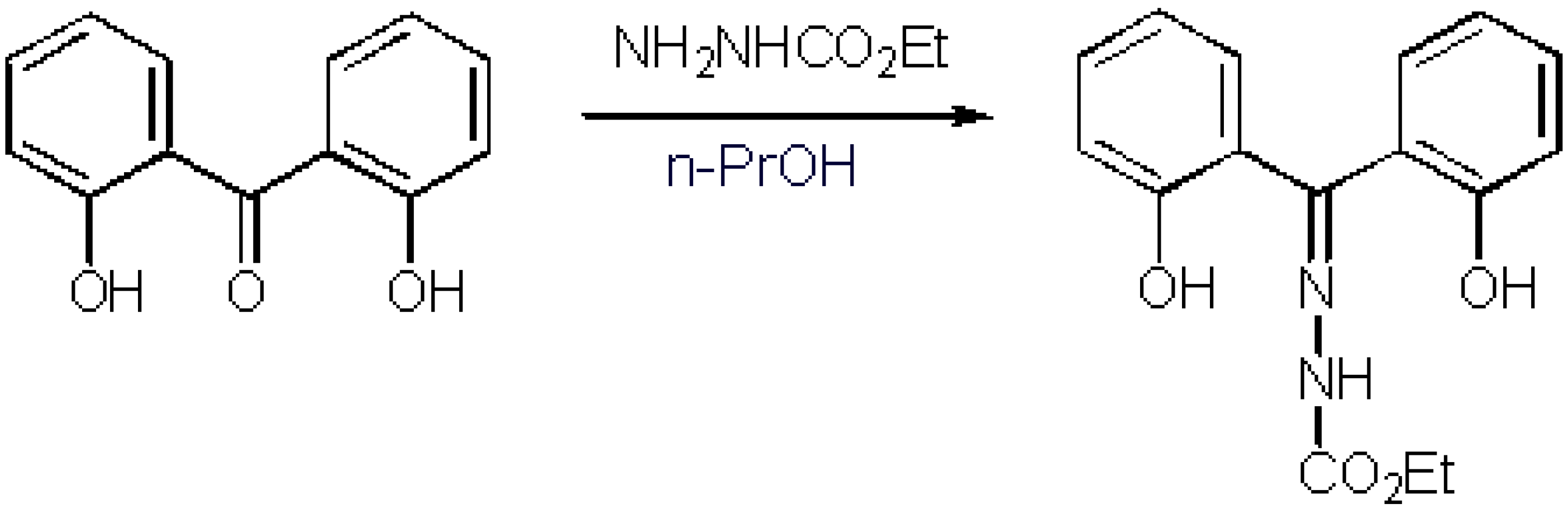
Both starting materials, ethylcarbazate as well as 2,2´-dihydroxybenzophenone, are commercially available and they were supplied by Aldrich. Ethylcarbazate (0.49 g, 4.7 mmol) was added to a solution of 2,2´-dihydroxybenzophenone (1 g, 4.67 mmol) in propanol-1 (10 mL). The reaction mixture was refluxed for 24 hours. It was then allowed to cool at room temperature. Subsequently, it was stored in the refrigerator overnight. The precipitation which was formed was then filtered to give the desired hydrazone (1.26 g, 90 %). The product was identified by 1H NMR, 13C NMR and MS and it was subjected to elemental analysis without further purification.
M.p. 177-179 °C.
1H NMR (300 MHz, DMSO-d6): 1.28 (t, 3H), 4.20 (q, 2H), 6.73-6.81 (m, 2H), 6.99-7.13 (m, 4H), 7.31-7.45 (m, 2H), 9.96 (s, 2H), 12.72 (s, 1H).
13C NMR (75 MHz, DMSO-d6): 14.4, 61.3, 116.4, 116.9, 117.2, 118.4, 119.5 (2 carbons), 129.5 (2 carbons), 130.5, 131.2, 152.6, 153.5, 154.7, 158.2.
MS m/z (AP+): 301 [M+1]+, 273, 255, 212.
Anal. Calc. for C16H16N2O4: C 63.99, H 5.37, N 9.33; found: C 64.05, H 5.43, N, 9.39.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Kotali, A.; Harris, P. A. Org. Prep. Proc. Int. 1994, 26, 159–192. [CrossRef]
- Kotali, A. Cur. Org. Chem. 2002, 6, 965–981.
- Sample availability: available from the authors and MDPI.
© 2003 MDPI. All rights reserved.