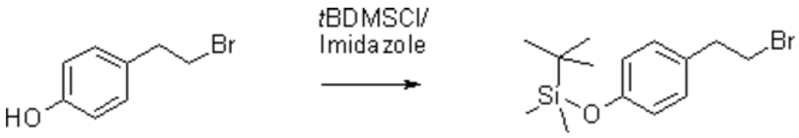
[4-(2-Bromoethyl)phenoxy]-(1,1-dimethylethyl)dimethylsilane
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Torssell, K.; Wahlberg, K. Isolation, structure, and synthesis of alkaloids from Valeriana officinalis. Acta Chem. Scand. 1967, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Available from the auhtors.
© 2003 MDPI. All rights reserved.
Share and Cite
Treu, M.; Jordis, U. [4-(2-Bromoethyl)phenoxy]-(1,1-dimethylethyl)dimethylsilane. Molbank 2002, 2002, M291. https://doi.org/10.3390/M291
Treu M, Jordis U. [4-(2-Bromoethyl)phenoxy]-(1,1-dimethylethyl)dimethylsilane. Molbank. 2002; 2002(1):M291. https://doi.org/10.3390/M291
Chicago/Turabian StyleTreu, Matthias, and Ulrich Jordis. 2002. "[4-(2-Bromoethyl)phenoxy]-(1,1-dimethylethyl)dimethylsilane" Molbank 2002, no. 1: M291. https://doi.org/10.3390/M291
APA StyleTreu, M., & Jordis, U. (2002). [4-(2-Bromoethyl)phenoxy]-(1,1-dimethylethyl)dimethylsilane. Molbank, 2002(1), M291. https://doi.org/10.3390/M291



