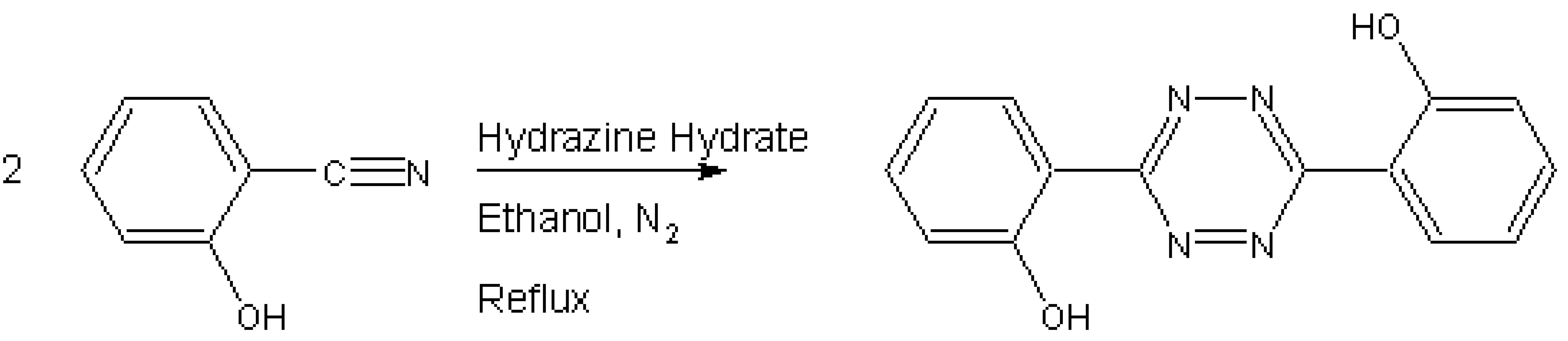
Hydrazine hydrate (99%, 5g, 100 mmol) was added to a solution of 2-cyanophenol (2 g, 16.8 mmol) in absolute ethanol (30 ml) under N2 atmosphere, and the reaction mixture was refluxed for 12 h. During the course of the reaction, the color of the solution changed to yellow, and the yellow precipitate, 2-[6-(2-hydroxyphenyl)-1,4-dihydro-1,2,4,5-tetrazin-3-yl]phenol, produced was readily oxidized in air to 2-[6-(2-hydroxyphenyl)-1,2,4,5- tetrazin-3-yl]phenol (0.9 g, 40% yield ), deep red crystals obtained from recrystallization from tetrahydrofuran.
1H-NMR (300MHz, CDCl3, δppm, JHz): 10.88 (2H, s), 8.66 (2H, d, J=8.1), 7.55 (2H, dd, J=7.2, 5.2), 7.16 (2H, d, J=7.2), 7.13 (2H, dd, J=8.2, 5.2).
IR (KBr, cm-1): 523 (m), 560 (w), 590 (w), 681 (s), 713 (s), 744 (s), 838 (m), 875 (w), 948 (m), 1029 (w), 1050 (m), 1094 (m), 1127 (w), 1155 (m), 1233 (s), 1273 (w), 1331 (w), 1386 (s), 1418 (s), 1472 (m), 1485 (s), 1577 (s), 1616 (s), 3119 (m).
UV-Vis (in CH3CN): 295 (e = 2.14 x 104 mol-1cm-1), 355 (e = 1.86 x 104 mol-1cm-1) and 534 nm (ε = 3.96 x 102 mol-1cm-1).
FAB-MS [M+]: 266.
Calcd: C, 63.15; H, 3.79; N, 21.04 %. Found: C, 63.0; H, 3.8; N, 21.1 %.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
Support for this research provided by the National Science Council (Taiwan) is gratefully acknowledged.
© 2003 MDPI. All rights reserved.