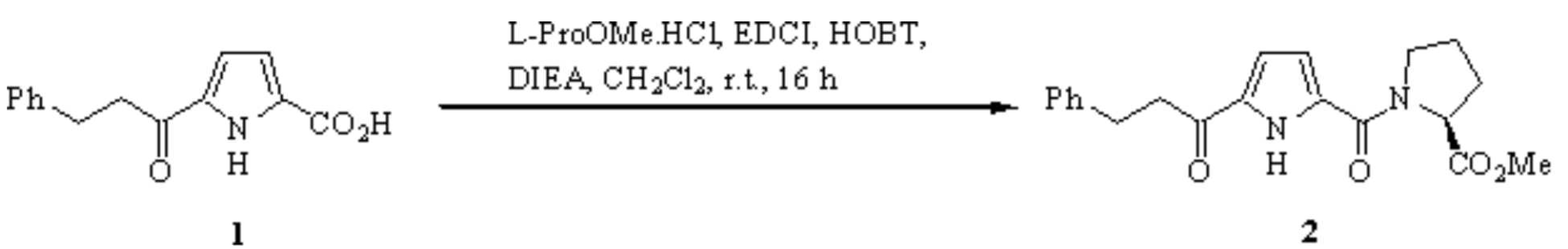
To a stirring solution of the pyrrole carboxylic acid 1 [1] (100 mg, 0.41 mmol, 1 equiv) and L-proline methyl ester hydrochloride (75 mg, 0.45 mmol, 1.1 equiv) in dry dichloromethane (6 mL) at r.t. under an inert atmosphere were added 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (102 mg, 0.53 mmol, 1.3 equiv) and 1-hydroxybenzotriazole hydrate (83 mg, 0.62 mmol, 1.5 equiv) [2].
N,N-Diisopropylethylamine (Hünig's base, 58 mg, 0.45 mmol, 1.1 equiv) was added, and the reaction mixture was stirred at r.t. for 16 h. The solution was then diluted with dichloromethane (10 mL), washed with 3 M hydrochloric acid (2 × 10 mL), water (2 × 10 mL), and the combined aqueous washings were back-extracted with dichloromethane (2 × 10 mL). The combined organic fractions were dried (MgSO4) and the solvent was removed by evaporation under reduced pressure. Flash chromatography on silica (ethyl acetate/petroleum ether, 2:1) afforded the title compound 2 (100 mg, 69%) as a tan solid.
mp 84-86°C.
IR (KBr, diffuse refraction method) 1539.1, 1598.9, 1660.6, 1737.7, 2954.7, 3028.0, 3435.0.
1H NMR (CDCl3, 500 MHz) d 2.03-2.27 (m, 4H, NCH2CH2CH2CH), 3.03 (m, 2H, PhCH2CH2), 3.11 (m, 2H, PhCH2CH2), 3.75 (s, 3H, CO2CH3), 3.84 (m, 1H, NCH2aCH2CH2CH), 3.96 (m, 1H, NCH2bCH2CH2CH), 4.68 (q, 1H, J = 4.1 Hz, NCH2CH2CH2CH), 6.61 (m, 1H, pyrrole H3), 6.82 (m, 1H, pyrrole H4), 7.17-7.29 (m, 5H, ArH), 10.10 (s (br), 1H, pyrrole NH).
13C NMR (CDCl3, 75 MHz) d 25.3, 28.6 (NCH2CH2CH2CH), 30.3 (PhCH2CH2), 40.1 (PhCH2CH2), 48.4 (NCH2CH2CH2CH), 52.4 (CO2CH3), 60.2 (NCH2CH2CH2CH), 112.9 (pyrrole C3), 115.4 (pyrrole C4), 126.2, 128.3, 128.5, 140.9 (ArC), 129.5 (pyrrole C2), 132.5 (pyrrole C5), 159.6 (CON), 172.5 (CO2), 189.9 (CH2CO).
HRMS (M+) Calcd for C20H22N2O4: 354.1580. Found: 354.1583.
Anal. Calcd for C20H22N2O4: C, 67.78; H, 6.26; N, 7.90. Found: C, 67.75; H, 6.51; N, 7.97.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Martyn, D. C.; Abell, A. D. J. Chem. Soc., Org. Biomol. Chem. in press.
- Tian, Z-Q.; Brown, B. B.; Mack, D. P.; Hutton, C. A.; Bartlett, P. A. J. Org. Chem. 1997, 62, 514.
- Sample availability: available from the authors.
© 2003 MDPI. All rights reserved.