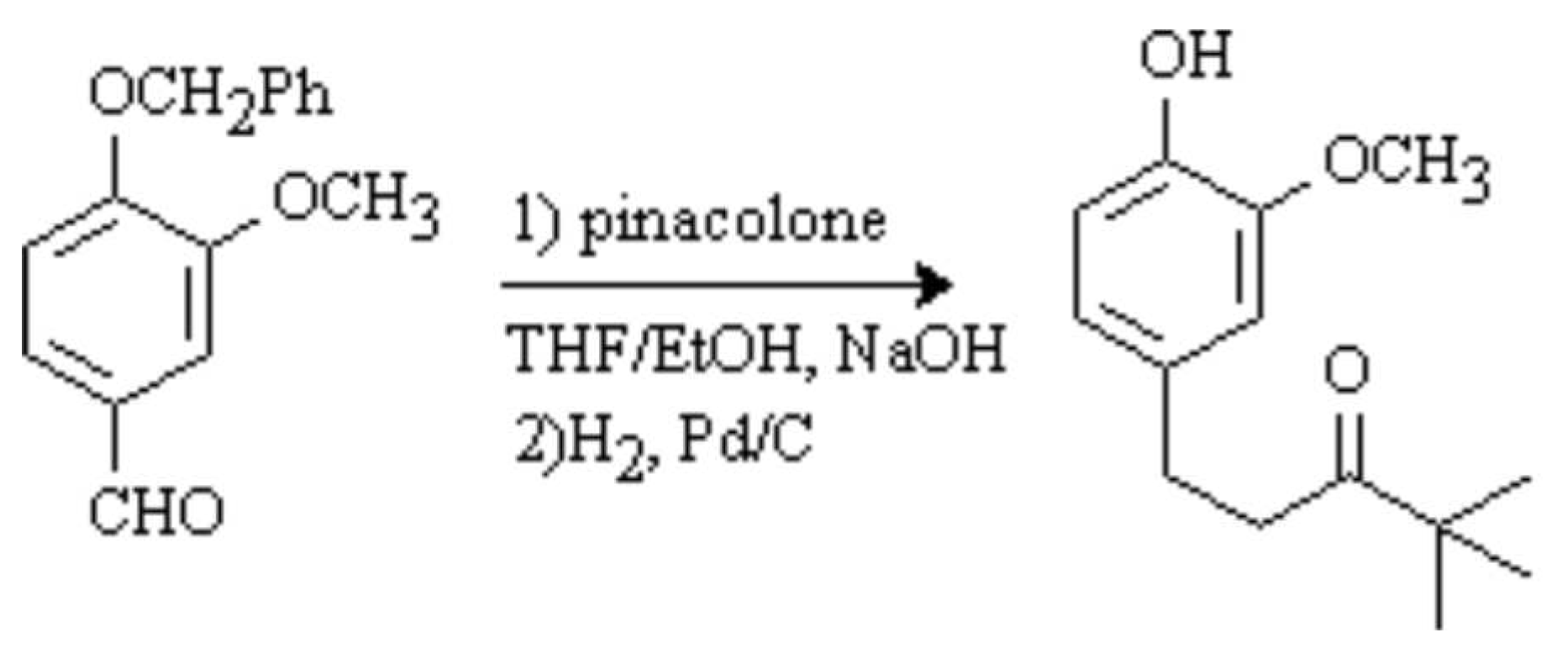
The discussion and purpose for the synthesis of this compound has been reported elsewhere [1]. To a solution of 4-benzyloxy-3-methoxybenzaldehyde (1.45 g, 6.0 mmol) in 50% THF/EtOH (100 mL) was added sodium hydroxide (0.5 g, 12.5 mmol, 2.1 eq) and pinacolone (2.0 g, 20 mmol, 3.3 eq) The resulting mixture was refluxed for 6 h (complete reaction by tlc). The solution was cooled to room temperature and 10% HCl (50 mL) was added. The mixture was concentrated in vacuo and the aqueous residue was extracted with dichloromethane (3 x 30 mL). The organic fractions were combined, dried (MgSO4) and the solvent was evaporated in vacuo to give a brown oil. The crude product was partially purified by chromatography on silica gel (20% EtOAc/hexanes) to give a yellow oil. This oil was dissolved in EtOAc (100 mL), Pd/C (135 mg) was added and the solution was stirred under a positve atmosphere of H2 for 20 h. The suspension was filtered through celite and the solvent was evaporated in vacuo. Chromatography on silica gel (20% EtOAc/hexanes) afforded a white solid (1.09 g, 77%).
mp: 65-66°C.
IR (KBr) cm-1: 3441 (OH), 1708 (CO).
1H-NMR (CDCl3) δ: 1.10 (s, 9H, CH3), 2.78 (m, 4H, H-1 and H-2), 3.87 (s, 3H, OCH3), 5.39 (s, 1H, exchangeable with D2O, OH), 6.62 (d, 1H, J=7.8 Hz, ArH-6), 6.69 (s, 1H, ArH-2), 6.83 (d, 1H, J=7.8 Hz, ArH-5).
13C-NMR (CDCl3) δ: 26.3 (CH3), 30.0 (C-2), 39.0 (C-1), 44.3 (C-4), 56.1 (OCH3), 111.2 (ArC-2), 114.5 (ArC-5), 121.0 (ArC-6), 133.8 (ArC-1), 144.0 (ArC-4), 146.5 (ArC-3), 215.4 (CO).
MS m/e (rel %): 236 [M+] (48), 179 (25), 151 (9), 137 (100).
Anal. calc. for C14H20O3 C 71.14, H 8.54, found C 71.32, H 8.49.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowlegment
The author is thankful for the financial support provided by the University of Northern British Columbia.
References
- Plourde, G.L. Tetrahedron Letters 2002, 43, 3597–3599.
© 2003 MDPI. All rights reserved.