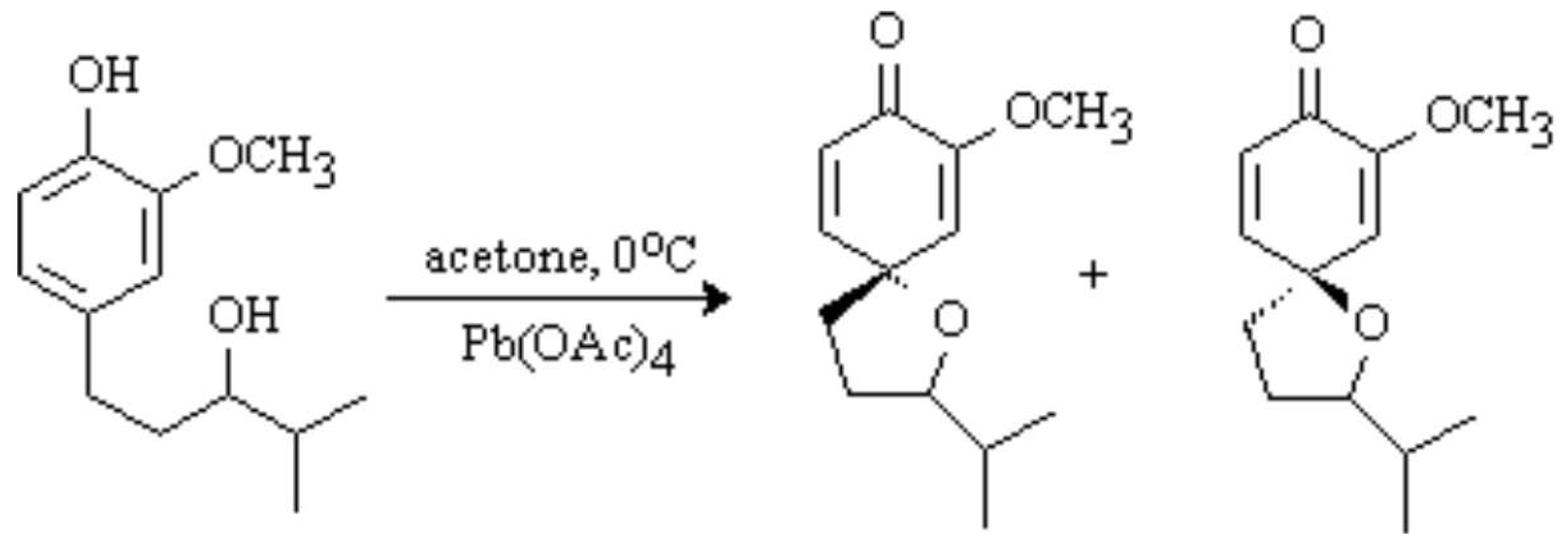
The discussion and purpose for the synthesis of this compound has been reported elsewhere [
1]. To a cold (0°C) solution of (±)-1-(4-hydroxy-3-methoxyphenyl)-4-methyl-3-pentanol (170 mg, 0.76 mmol) in acetone (20 mL) was added in one portion Pb(OAc)
4 (1.2 g, 2.7 mmol, 3.6 eq). The resulting orange mixture was stirred at 0°C for 2 h. The precipitate was filtered through celite and ethylene glycol (10 drops) was added. The solution was stirred at room temperature for 20 h and filtered through celite. The solvent was evaporated in vacuo to afford a racemic mixture of diastereomers (71/29 ratio).
Chromatography on silica gel (30% EtOAc/hexanes) afforded 3 fractions [total of 139 mg (83%)], 26 mg as the diastereomeric mixture, 44 mg of the minor isomer as a clear oil, and 72 mg of the major isomer as a white solid (mp: 77-78°C).
IR cm-1: Major (KBr): 1676 (CO), Minor (neat): 1682 (CO).
1H-nmr (CDCl3) d: Major: 0.94 (d, 3H, J=6.8 Hz, CH3), 1.01 (d, 3H, J=6.6 Hz, CH3), 1.79 (m, 2H, H-3), 2.15 (m, 3H, H-4 and isopropyl CH), 3.69 (s, 3H, OCH3), 3.96 (m, 1H, H-2), 5.76 (d, 1H, J=2.7 Hz, H-6), 6.12 (d, 1H, J=10.0 Hz, H-9), 6.79 (dd, 1H, J=2.7, 10.0 Hz, H-10); Minor: 0.94 (d, 1H, J=6.8 Hz, CH3), 1.00 (d, 1H, J=6.7 Hz, CH3), 1.81 (m, 2H, H-3), 2.20 (m, 3H, H-4 and isopropyl CH), 3.72 (s, 3H, OCH3), 3.90 (m, 1H, H-2), 5.67 (d, 1H, J=2.6 Hz, H-6), 6.13 (d, 1H, J=9.9 Hz, H-9), 6.87 (dd, 1H, J=2.6, 9.9 Hz, H-10).
13C-nmr (CDCl3) d: Major: 18.6 (CH3), 19.5 (CH3), 29.9 (C-3), 33.6 (ipropyl CH), 38.3 (C-4), 55.1 (OCH3), 79.6 (C-5), 86.5 (C-2), 117.4 (C-6), 126.2 (C-9), 149.9 (C-7), 151.2 (C-10), 181.3 (CO); Minor: 18.6 (CH3), 19.4 (CH3), 29.7 (C-3), 33.4 (ipropyl CH), 37.9 (C-4), 55.0 (OCH3), 79.5 (C-5), 86.2 (C-2), 117.6 (C-6), 126.2 (C-9), 149.7 (C-7), 151.0 (C-10), 181.2 (CO).
MS m/e (rel %): Major: 222 [M+] (32), 179 (32), 164 (66), 153 (100), 140 (74), 119 (75), 91 (53); Minor: 222 [M+] (22), 179 (4), 166 (5), 153 (100), 125 (6), 91 (2).
Anal. calc. for C13H18O3: C 70.23, H 8.17; found: C 70.12, H 8.21.