Abstract
A novel carbamate prodrug 2 containing a pentagastrin moiety was synthesized. 2 was designed as a detoxified analogue of the highly cytotoxic natural antibiotic duocarmycin SA (1) for the use in a targeted prodrug monotherapy of cancers expressing cholecystokinin (CCK-B)/gastrin receptors. The synthesis of prodrug 2 was performed using a palladium-catalyzed carbonylation of bromide 6, followed by a radical cyclisation to give the pharmacophoric unit 10, coupling of 10 to the DNA-binding subunit 15 and transformation of the resulting seco-drug 3b into the carbamate 2 via addition of a pentagastrin moiety.
1. Introduction
One of the major problems in the chemotherapy of cancers is the usually low differentiation between normal and malignant cells by the known antiproliferating agents, resulting in severe side effects. Several approaches have been developed to overcome this problem like the antibody-directed enzyme prodrug therapy (ADEPT) [1,2] and the prodrug monotherapy (PMT) [3]. Whereas in ADEPT artificial antibody-enzyme conjugates are needed for targeting tumor cells, in PMT specific endogenous enzymes or receptors overexpressed in cancerous tissue are addressed to allow a selective killing of tumor cells. In both approaches, a relatively untoxic prodrug is used, which is then selectively converted into the corresponding cytotoxic drug in the cancer tissue; however, for PMT the prodrug is linked to a ligand which allows a targeting of cancer cells. Among these ligands small peptides play an important role having a low immunogenicity as well as high specifities and affinities to certain receptors which are overexpressed on certain tumour cells [4]. Some of these peptides that are already successfully applied in cancer therapy, belong to the gastrin family. For example, radiolabeled gastrin derivatives have shown a high therapeutic and diagnostic potential in targeting cholecystokinin (CCK-B)/gastrin receptor expressing tumors [5]. In addition, a gastrin derivative was linked to a triazene alkylating agent [6]. However, the observed receptor-mediated cytotoxicity of this conjugate was quite low. Better results were obtained with heptagastrin linked to an ellipticine derivative [7]. A high receptor-mediated cytotoxicity could be achieved with the anthracyclines daunorubicin, doxorubicin and 2-pyrrolinodoxorubicin as well as other cytotoxic agents like melphalan, cisplatin or methotrexate coupled to peptides of the LHRH [4,8], bombesin [4,8a,9], somatostatin [4,8a,10] and neuropeptide Y [4,11] type. Recently, we have developed the pentagastrin-toxin conjugate 3a containing a seco-duocarmycin SA derivative (Scheme 1) [12].
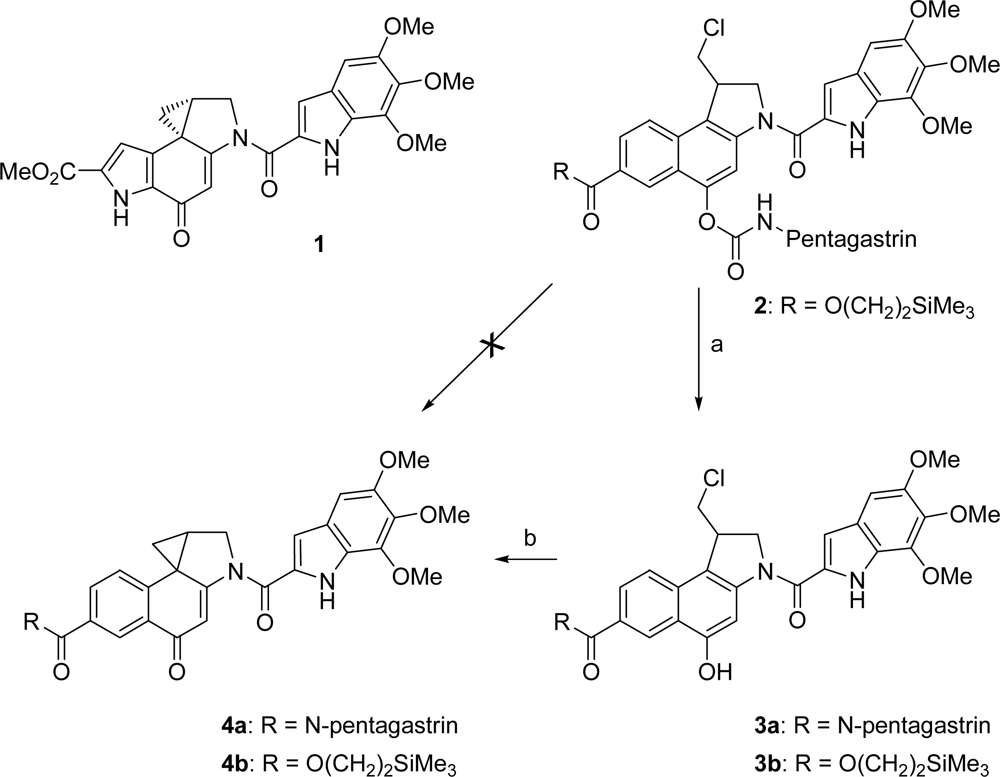
Scheme 1.
(+)-Duocarmycin SA (1), prodrug 2, seco-drugs 3 and drugs 4; a) enzymatic toxification of the novel carbamate prodrug 2 to the seco-drug 3b inside the tumor cells and b) rapid cyclisation of the seco-drugs 3 in situ to give the cytotoxic drugs 4.
Here, we report the synthesis of a novel pentagastrin conjugate 2 for the use in a targeted tumor therapy, which has the advantage over normal gastrin-toxin conjugates that a prodrug is used instead of a toxic drug (Scheme 1). In this concept, the pentagastrin moiety should serve not only as a targeting ligand for CCK-B/gastrin receptors, but also as a detoxifying unit. Thus, the corresponding drug 4b, which again is an analogue of the naturally occuring antibiotic (+)-duocarmycin SA (1) with an IC50 value of 10 pm (L1210) [13], should be formed via 3b inside the tumor cells by cleavage of the carbamate by lysosomal enzymes after endocytosis. The antiproliferative effect of 1 and its analogues such as the CBI-drugs 4 derive most probably from a selective alkylation of N-3 of adenine in DNA by nucleophilic attack at the spirocyclopropyl-cyclohexadienone moiety as the pharmacophoric group [14]. Since we have previously shown that the formation of a drug as 4b from a seco-drug as 3b is a very fast process and that the blocking of the phenolic hydroxyl group of 3b allows a very strong reduction of its cytotoxicity, we used the seco-drug 3b as substrate for the conjugation with the pentagastrin moiety, performing the connection to the phenolic hydroxyl group via a carbamate moiety [1b,1c,15].
In our approach we did not employ the whole heptadecapeptide gastrin but the shorter β-alanine modified pentagastrin, because its β-Ala-Trp-Met-Asp-Phe-NH2 sequence representing the C-terminal amide of the natural peptides restores the biological activity of gastrin in a comparable order of magnitude [4e,16]. As a consequence, the seco-duocarmycin moiety 3b had to be attached via the N-terminal amino functionality of pentagastrin using a carbamate. Such a carbamate substructure exists also in KW-2189 (5) (Figure 1) [17], an agent already investigated in clinical trials, and in several other anticancer agents [18].
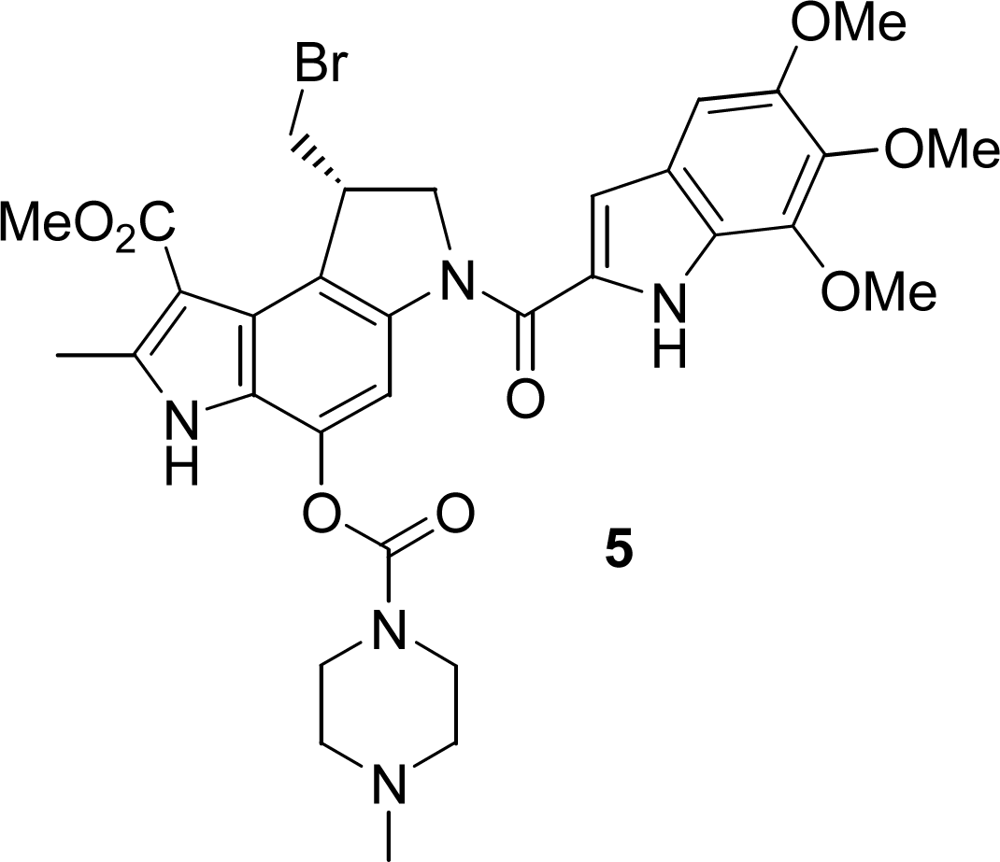
Figure 1.
KW-2189 (5).
For the formation of the carbamate moiety, we envisaged an addition of an isocyanate to the seco-drug 3b. The TMSE ester moiety was introduced to allow a better comparison with the already prepared pentagastrin-conjugate 3a. Moreover, the handle could be used for the introduction of a fluorescence dye to allow an investigation of the mode of action of such a compound employing a confocal laser scanning microscope.
2. Results and Discussion
2.1. Synthesis
As starting material for the preparation of 2 we employed the known aminonaphthalin 6 [12]. 6 was converted into TMSE ester 7 in 56 % yield by a palladium-catalyzed carbonylation reaction using a CO atmosphere (1 bar) and Mo(CO)6 as additional CO source [19] in a mixture of 2-(trimethylsilyl)-ethanol and DMF (Scheme 2). The moderate yield of 56% of this carbonylation reaction might be due to the relatively high electron density of 6. Nevertheless, 6 had to be used in the carbonylation reaction as the Curtius rearrangement of the corresponding acid to the protected naphtholamine could not be achieved after the introduction of the TMSE ester moiety. Iodination of 7 employing NIS [20,21] with TsOH·H2O as catalyst followed by N-alkylation of the formed 8 with 1,3-dichloropropene and subsequent radical cyclization [22] using the untoxic tris-(trimethylsilyl)-silan (TTMSS) [23] as hydride source and AIBN as radical starter provided seco-CBI derivative 10 in 65 % yield over three steps.
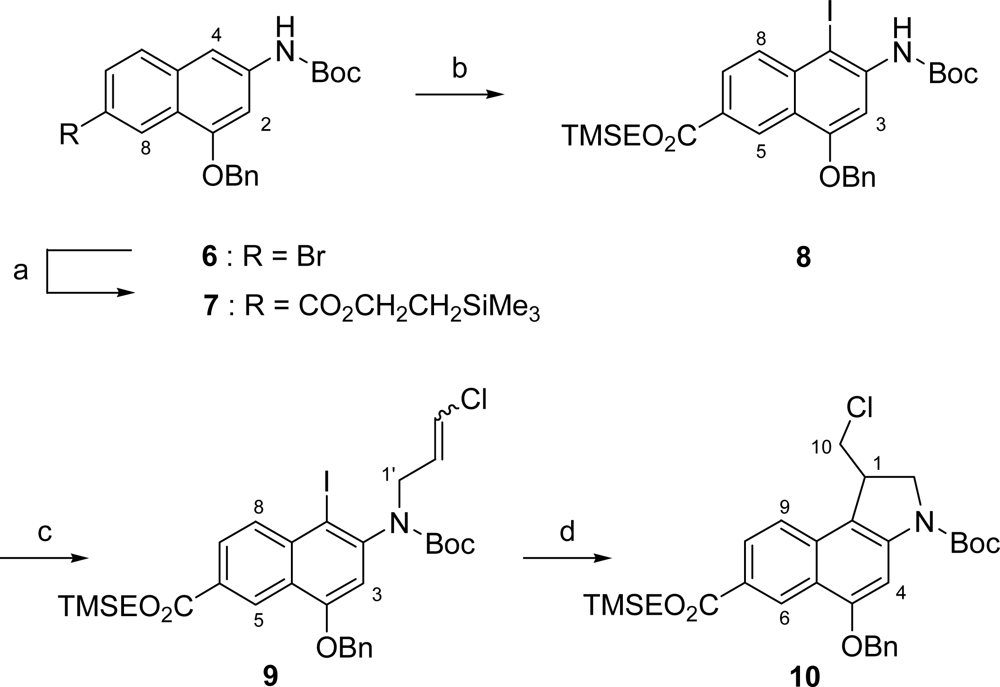
Scheme 2.
Synthesis of seco-CBI compound 10. a) Mo(CO)6, 1 bar CO, 5 mol% Pd(PPh3)2Br2, 20 mol% dppf, nBu3N, TMSEOH, DMF, 120 °C, 7 h, 56%; b) NIS, TsOH·H2O, THF/MeOH, 50 °C, 1 h, 73%; c) NaH, 1,3-dichloropropene, DMF, 20 °C, 13.5 h, 97%; d) HSi(SiMe3)3, AIBN, benzene, reflux, 2 h, 92%.
In order to connect 10 with the peptide unit, we used the isocyanate 13 containing an ester moiety. This was first reacted with the phenolic hydroxyl group and then bound to the peptide via an amide linkage. The required isocyanate 13 was prepared as follows: first, β-alanine (11) was converted into the corresponding benzyl ester hydrochloride 12 employing TMSCl and benzylic alcohol [24]. Then, the isocyanate moiety was introduced using solid and thus easy to handle triphosgene in refluxing toluene to give 13 in 87 % yield over two steps (Scheme 3).
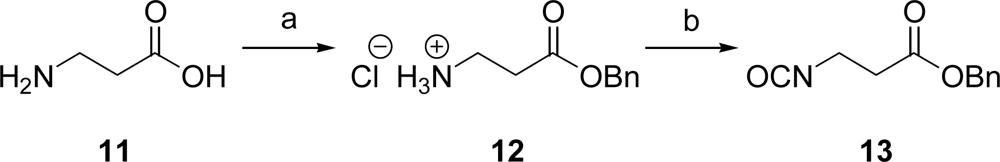
Scheme 3.
Synthesis of isocyanate 13. a) TMSCl, BnOH, 20 °C, 15 h, 89%; b) triphosgene, toluene, reflux, 7.5 h, 98%.
Hence, the synthesis of carbamate prodrug 2 was completed in seven further steps (Scheme 4). After deprotection of the secondary amino functionality in 10 under acidic conditions in an aqueous HCl/EtOAc mixture with Et3SiH as cation scavenger [25], the obtained hydrochloride salt 14 was directly coupled with the DNA-binding subunit TMI-CO2H (15) to give 16 in 43 % yield over two steps. Then, the benzyl ether moiety in 16 was cleaved by transfer hydrogenolysis with an aqueous ammonium formate solution and palladium on charcoal as the catalyst [26] to yield phenol 3b which was subsequently coupled with isocyanate 13 to afford carbamate 17 in a very good yield of 83 % over two steps. The benzyl ester in 17 was cleaved again by using transfer hydrogenolytic conditions to give 18 in 90 % yield. This reaction had to be carefully monitored by TLC as the carbamate was sensitive to these conditions. Finally, carboxylic acid 18 was treated with HOSu/EDC·HCl and the resulting active ester 19 directly coupled with the fully unprotected tetrapeptide 20 [27] to yield carbamate prodrug 2 in 57 % (83 % based on recovered starting material) over two steps.
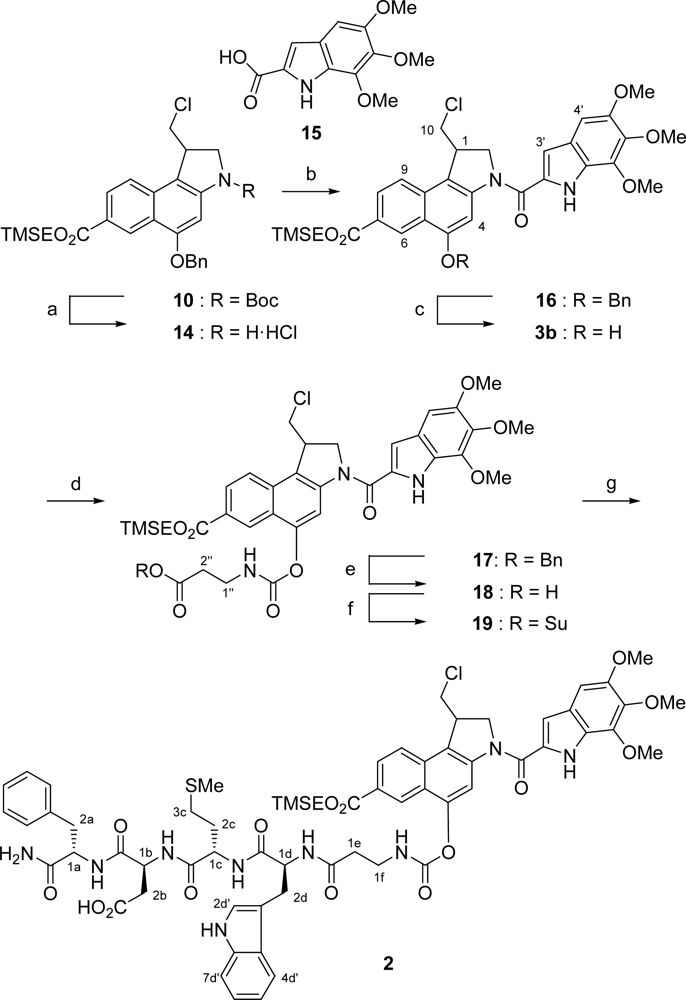
Scheme 4.
Synthesis of carbamate prodrug 2. a) HCl/EtOAc, Et3SiH, CH2Cl2, 20 °C, 7 h; b) TMI-CO2H (15), EDC·HCl, DMF, 20 °C, 1 d, 43% (two steps); c) Pd/C, NH4HCO2, THF/MeOH, 20 °C, 75 min, quant.; d) isocyanate 13, NEt3, CH2Cl2, 0–20 °C, 16 h, 83%; e) Pd/C, NH4HCO2, THF/MeOH, 20 °C, 25 min, 90%; f) HOSu, EDC·HCl, THF/CH2Cl2, 0–20 °C, 15 h; g) tetragastrin (20), NEtiPr2, H2O/DMF, 20 °C, 7 h, 57% (two steps).
2.2. In vitro cytotoxicity tests
The in vitro cytotoxicity assays were carried out in duplicate with CCK-B/gastrin-receptor positive cells of the human pancreatic cell line MIA PaCa-2 and CCK-B/gastrin-receptor negative cells of the human bronchial carcinoma cell line A549 as control in six multiwell plates with concentrations of 102, 103 and 104 cells per cavity. Incubation with various concentrations of the seco-drug 3b and the prodrug 2 was performed in ultraculture medium (Table 1).

Table 1.
In vitro cytotoxicity of prodrug 2 and of seco-drug 3b against CCK-B/gastrin-receptor positive cells of the human pancreatic cell line MIA PaCa-2 and CCK-B/gastrin-receptor negative human bronchial carcinoma cells (A549). Cells were exposed to various concentrations of the test substance for 24 h at 37 °C; after 10 days of incubation following the exposure to the substance, clone formation was compared to an untreated control assay and the relative colony-forming rate was determined. IC50 is the drug concentration required for 50% growth inhibition of target cells.
Prodrug 2 shows the same cytotoxicity as its corresponding seco-drug 3b in the cell culture assays using the CCK-B/gastrin-receptor positive cell line (MIA PaCa) and the CCK-B/gastrin-receptor negative cell line (A549). Thus, the obtained IC50-values are almost identical in these four experiments. This indicates that prodrug 2 seems not to be stable under the used cell culture conditions. In fact, HPLC-MS-measurements revealed a decomposition of prodrug 2 under loss of the targeting pentagastrin moiety thereby forming the corresponding seco-drug 3b.
We suppose that the unstability of the carbamate moiety can be traced back to the hydrogen atom at its nitrogen which in turn is part of the β-alanine moiety of pentagastrin. We therefore plan to replace the hydrogen by a carbon moiety though it is not known whether such a modification of the pentagastrin would interfere with the binding of the conjugate to the corresponding CCK-B/gastrin-receptor.
3. Experimental Section
General: All reactions were performed in flame dried glassware under an argon atmosphere. Solvents were dried and purified according to standard procedures and redistilled prior to use. TLC chromatography was performed on precoated aluminium silica gel SIL G/UV254 plates (Macherey-Nagel & Co.) and silica gel 60 (0.040-0.063 mm) (Merck) was used for column chromatography. IR: Bruker Vector 22. UV/VIS: Perkin-Elmer Lambda 2. 1H-NMR: Varian Mercury-200, Unity-300 (300 MHz), Unity Inova-600 (600 MHz). 13C-NMR: Varian Mercury-200 (50 MHz), Unity-300 (75 MHz), Unity Inova-600 (150 MHz). For 1H and 13C, CDCl3, [D6]DMSO and [D7]DMF were used as solvents. Chemical shifts are reported on a δ scale. Signals are quoted as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), mc (centered multiplet) and br (broad). MS: Finnigan MAT 95, TSQ 7000, LCQ. HRMS was performed using among others a modified peak matching technique, error ±2 ppm, with a resolution of ca. 10,000. Elemental analysis: Mikroanalytisches Labor des Institutes für Organische und Biomolekulare Chemie der Universität Göttingen.
3-Amino-1-benzyloxy-N-(tert-butoxycarbonyl)-7-[2-(trimethylsilyl)-ethoxycarbonyl]-naphthalene (7): A magnetically stirred and degassed solution of bromide 6 (3.60 g, 8.40 mmol), N(nBu)3 (6.0 ml, 4.7 g, 25 mmol) and 2-(trimethylsilyl)-ethanol (6.0 ml, 5.0 g, 42 mmol) in DMF (35 ml) was treated with Pd(PPh3)2Br2 (332 mg, 420 μmol), 1,1'-bis-(diphenylphosphino)-ferrocene (931 mg, 1.68 mmol) and Mo(CO)6 (1.1 g, 4.2 mmol). The reaction mixture was degassed again, set under a carbon monoxide atmosphere (1 bar) and stirred for 7 h at 120 °C (preheated bath). The alcohol and tributylamine were distilled off under reduced pressure, the resulting red oil adsorbed on silica gel and subjected to column chromatography (pentane/EtOAc = 10:1 → 7:1) to afford ester 7 (2.32 g, 56 %) as orange solid. Rf = 0.43 (pentane/EtOAc = 7:1); 1H NMR (300 MHz, CDCl3): δ = 0.08 (s, 9 H, Si(CH3)3), 1.13–1.18 (m, 2 H, CH2SiMe3), 1.55 (s, 9 H, C(CH3)3), 4.43–4.49 (m, 2 H, CH2OC=O), 5.26 (s, 2 H, CH2Ph), 6.73 (brs, 1 H, NH), 7.08 (d, J = 1.5 Hz, 1 H, 2-H), 7.34–7.64 (m, 6 H, 5 × Ph-H, 4-H), 7.69 (d, J = 8.7 Hz, 1 H, 5-H), 8.02 (dd, J = 8.7, 2.1 Hz, 1 H, 6-H), 8.96 ppm (d, J = 2.1 Hz, 1 H, 8-H); 13C NMR (50 MHz, CDCl3): δ = –1.40 (Si(CH3)3), 17.35 (CH2SiMe3), 28.32 (C(CH3)3), 63.09 (CH2OC=O), 70.30 (CH2Ph), 80.97 (C(CH3)3), 99.48 (C-3), 106.31 (C-1), 121.59, 125.61 (C-4a, C-6), 125.31, 126.61, 126.87, 127.45, 128.04, 128.61 (C-5, C-7, C-8, 5 × Ph-C), 136.41, 137.13, 138.65 (C-2, C-8a, Ph-Ci), 152.51, 156.23 (C-4, C=O), 167.11 ppm (C(O)OTMSE); MS (EI, 70 eV): m/z (%) = 493 (18) [M]+, 437 (10) [M – C4H9 + H]+, 91 (100) [C7H7]+.
2-Amino-4-benzyloxy-N-(tert-butoxycarbonyl)-1-iodo-6-[2-(trimethylsilyl)-ethoxycarbonyl]-naphthalene (8): A magnetically stirred solution of 7 (352 mg, 713 μmol) in 1:1 THF/MeOH (10 ml) was treated with a solution of TsOH·H2O (14 mg, 71 μmol) in THF (1 mL) and N-iodosuccinimide (322 mg, 1.43 mmol). The resulting mixture was warmed to 50 °C and stirred for 1 h. The reaction mixture was quenched with NaHCO3 (5 ml, saturated solution) and water and extracted with EtOAc (2 × 10 ml). The combined organic phases were washed with Na2S2O3 (1 × 15 ml, saturated solution) and brine (15 ml), dried (MgSO4) and concentrated under reduced pressure to give an orange oil. This material was adsorbed on silica gel and subjected to column chromatography (pentane/EtOAc = 20:1) to afford iodide 8 (320 mg, 73 %) as colorless foam. Rf = 0.65 (pentane/EtOAc = 10:1); 1H NMR (300 MHz, CDCl3): δ = 0.08 (s, 9 H, Si(CH3)3), 1.13–1.18 (m, 2 H, CH2SiMe3), 1.59 (s, 9 H, C(CH3)3), 4.44–4.49 (m, 2 H, CH2OC=O), 5.31 (s, 2 H, CH2Ph), 7.33–7.60 (m, 5 H, 5 × Ph-H), 8.04 (d, J = 9.3 Hz, 1 H, 8-H), 8.09 (dd, J = 9.3, 1.8 Hz, 1 H, 7-H), 8.14 (s, 1 H, 3-H), 8.95 ppm (d, J = 1.8 Hz, 1 H, 5-H); 13C NMR (50 MHz, CDCl3): δ = –1.40 (Si(CH3)3), 17.34 (CH2SiMe3), 28.30 (C(CH3)3), 63.29 (CH2OC=O), 70.55 (CH2Ph), 79.16 (C(CH3)3), 81.53 (C-1), 100.13 (C-3), 122.77, 126.27, 136.16, 137.06 (C-4a, C-6, C-8a, Ph-Ci), 125.62, 127.90, 128.07, 128.16, 128.58, 131.39 (C-5, C-7, C-8, 5 × Ph-C), 140.53 (C-2), 152.51, 156.60 (C-4, C=O), 166.66 ppm (C(O)OTMSE); MS (EI, 70 eV): m/z (%) = 619 (14) [M]+, 563 (10) [M – C4H9 + H]+, 535 (18) [M – C4H8 – CO]+, 491 (5) [M – I – H]+, 91 (100) [C7H7]+, 57 (36) [C4H9]+.
(E/Z)-2-Amino-4-benzyloxy-N-(tert-butoxycarbonyl)-N-(3-chloro-2-propenyl)-1-iodo-6-[2-(trimethylsilyl)-ethoxycarbonyl]-naphthalene (9): A magnetically stirred solution of 8 (54 mg, 87 μmol) in DMF (1.5 ml) was treated with NaH (5.20 mg, 60 % in oil, 218 μmol). Stirring was continued for 40 min at 20 °C before (E/Z)-1,3-dichloropropene (16.0 μl, 19.0 mg, 174 μmol) was added dropwise, and it was stirred for a further 13 h at 20 °C. The ensuing mixture was then adjusted to pH 5 with NH4Cl (saturated solution) and extracted with EtOAc (4 × 5 mL). The combined organic phases were washed with water (10 ml), brine (10 ml), dried (MgSO4) and concentrated under reduced pressure to give an orange oil. Subjection of this material to column chromatography (pentane/EtOAc = 10:1) gave iodide 9 (59 mg, 97 %) as pale yellow foam. Rf = 0.43, 0.52 (pentane/EtOAc = 10:1); 1H NMR (200 MHz, CDCl3): δ = –0.14/0.10 (2 × s, 9 H, Si(CH3)3), 1.14–1.22 (m, 2 H, CH2SiMe3) 1.30/1.58 (2 × s, 9 H, C(CH3)3), 3.78 (dd, J = 13.8, 6.4 Hz, 1 H, 1′-Ha), 4.19–4.35 (m, 1 H, 1′-Hb), 4.46–4.54 (m, 2 H, CH2OC=O), 5.31 (brs, 2 H, CH2Ph), 5.92–6.15 (m, 2 H, 2′-H, 3′-H), 6.65–6.85 (m, 1 H, 3-H), 7.30–7.55 (m, 5 H, 5 × Ph-H), 8.15 (d, J = 8.0 Hz, 1 H, 8-H), 8.25 (d, J = 8.0 Hz, 1 H, 7-H), 9.01–9.10 ppm (m, 1 H, 5-H); 13C NMR (50 MHz, CDCl3): δ = –1.40 (Si(CH3)3), 17.33 (CH2SiMe3), 28.19/28.46 (C(CH3)3), 45.76/48.97 (C-1′), 63.53/64.59 (CH2OC=O), 70.49/70.58 (CH2Ph), 80.88/81.39 (C(CH3)3), 94.52 (C-1), 107.93/108.51 (C-3), 120.87/121.96 (C-3′), 124.79, 128.26/128.29, 135.91/136.01, 137.51/137.64 (C-4a, C-6, C-8a, Ph-Ci), 125.36, 127.00/127.20, 127.90/127.94, 128.17/128.21, 128.45, 128.72/128.76, 133.06 (C-5, C-7, C-8, C-2′, 5 × Ph-C), 144.60/144.98 (C-2), 153.37/153.59 (C-4), 155.99/156.12 (C=O), 166.40/166.43 ppm (C(O)OTMSE); MS (EI, 70 eV): m/z (%) = 694 (16) [M + H]+, 566 (18) [M – I]+, 510 (88) [M – I – C4H9 + H]+, 91 (100) [C7H7]+, 57 (16) [C4H9]+.
(1R/S)-5-Benzyloxy-3-(tert-butoxycarbonyl)-1-chloromethyl-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (10): Through a magnetically stirred solution of iodide 9 (308 mg, 444 μmol) in benzene (13 ml) was bubbled argon for 45 min. The oxygen-free solution was then treated with tris-(trimethylsilyl)-silane (124 μl, 99.0 mg, 400 μmol) and AIBN (17.0 mg, 102 μmol) and stirred for 2 h under reflux. The ensuing mixture was adsorbed on silica gel and subjected to column chromatography (pentane/EtOAc = 10:1) to afford 10 (208 mg, 92 %) as pale yellow solid. Rf = 0.44 (pentane/EtOAc = 10:1); UV/VIS (CH3CN): λmax (lg ε) = 217 (4.417), 271 (4.753), 354 nm (4.158); IR (KBr): ν̃ = 3388 (NH), 2955, 1700 (C=O), 1621, 1461, 1412, 1366, 1249, 1139, 928, 839 cm–1; 1H NMR (300 MHz, CDCl3): δ = 0.08 (s, 9 H, Si(CH3)3), 1.12–1.18 (m, 2 H, CH2SiMe3), 1.61 (s, 9 H, C(CH3)3), 3.45 (t, J = 10.5 Hz, 1 H, 10-Hb), 3.88–4.02 (m, 2 H, 1-H, 10-Ha), 4.14 (dd, J = 11.4, 9.0 Hz, 1 H, 2-Hb), 4.27 (d, J = 11.4 Hz, 1 H, 2-Ha), ), 4.44–4.49 (m, 2 H, CH2OC=O), 5.29 (s, 2 H, CH2Ph), 7.35–7.48 (m, 3 H, 3 × Ph-H), 7.54–7.59 (m, 2 H, 2 × Ph-H), 7.64 (d, J = 8.7 Hz, 1 H, 9-H), 7.87 (brs, 1 H, 4-H), 8.08 (dd, J = 8.7, 1.5 Hz, 1 H, 8-H), 9.02 ppm (d, J = 1.5 Hz, 1 H, 6-H); 13C NMR (125 MHz, CDCl3): δ = –1.41 (Si(CH3)3), 17.33 (CH2SiMe3), 28.41 (C(CH3)3), 41.38 (C-1), 46.36 (C-10), 53.11 (C-2), 63.14 (CH2OC=O), 70.42 (CH2Ph), 81.43 (C(CH3)3), 96.93 (C-4), 114.37, 121.50, 124.92, 132.37, 144.14 (C-3a, C-5a, C-7, C-9a, C-9b), 121.72 (C-9), 126.75 (C-6), 127.21 (C-8), 127.62, 128.07, 128.59 (5 × Ph-C), 136.34 (Ph-Ci), 152.41 (C-5), 157.25 (C=O), 166.91 ppm (C(O)OTMSE); MS (EI, 70 eV): m/z (%) = 567 (4) [M]+, 511 (7) [M – C4H9 + H]+, 91 (90) [C7H7]+, 57 (30) [C4H9]+; HRMS: calcd for C31H38ClNO5Si: 567.2208; confirmed.
β-Alanine benzyl ester hydrochloride (12): A magnetically stirred suspension of β-alanine (11) (1.00 g, 11.2 mmol) in benzylic alcohol (56.0 ml, 58.0 g, 539 mmol) was treated dropwise over a period of 10 min with trimethylsilylchloride (3.6 ml, 3.0 g, 28 mmol) and stirring continued for a further 15 h at 20 °C. The resulting clear solution was poured into Et2O (600 mL), the precipitate collected by filtration and washed with Et2O (100 mL). Drying of this material under reduced pressure gave hydrochloride 12 (2.15 g, 89 %) as white solid; 1H NMR (200 MHz, [D6]DMSO): δ = 2.79 (t, J = 7.0 Hz, 2 H, 2-H2), 3.03 (t, J = 7.0 Hz, 2 H, 3-H2), 5.13 (s, 2 H, CH2Ph), 7.32–7.41 (m, 5 H, 5 × Ph-H), 8.23 ppm (brs, 3 H, NH3+); 13C NMR (50 MHz, [D6]DMSO): δ = 31.34 (C-2), 34.47 (C-3), 65.87 (CH2Ph), 127.95 (2 × Ph-C), 128.01 (Ph-Cp), 128.34 (2 × Ph-C), 135.73 (Ph-Ci), 170.05 ppm (C=O); MS (ESI): m/z (%) = 180 (100) [M – Cl]+, 359 (100) [2M – Cl – HCl]+.
3-Isocyano-propionic acid benzyl ester (13): A magnetically stirred suspension of hydrochloride 12 (2.13 g, 9.88 mmol) in toluene (15 ml) was treated with triphosgene (2.93 g, 9.88 mmol) and heated to reflux for 7.5 h (end of HCl-evolution). The resulting solution was concentrated under reduced pressure to afford isocyanate 13 (1.99 g, 98 %) as yellow liquid which was used for the next reaction without further purification. Rf = 0.21 (pentane/EtOAc = 10:1); IR (film): ν̃ = 3349, 2957, 2277 (NCO), 1736 (C=O), 1498, 1176, 823, 752, 699 cm–1; 1H NMR (200 MHz, CDCl3): δ = 2.65 (t, J = 6.4 Hz, 2 H, 2-H2), 3.61 (t, J = 6.4 Hz, 2 H, 3-H2), 5.18 (s, 2 H, CH2Ph), 7.35–7.40 ppm (m, 5 H, 5 × Ph-H).
(1R/S)-5-Benzyloxy-1-chloromethyl-3-(5,6,7-trimethoxyindole-2-carbonyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (16): A solution of the protected amine 10 (369 mg, 650 μmol) in CH2Cl2 (5 ml) was treated with HCl (18 ml of a 4 m solution in EtOAc) and Et3SiH (105 μl, 76.0 mg, 650 μmol) and stirred for 7 h at 20 °C. The solvent was removed under reduced pressure and the ensuing residue treated with toluene (2 × 10 ml) and again concentrated under reduced pressure. The resulting crude hydrochloride 14 was dried under reduced pressure and then treated with 5,6,7-trimethoxyindole-2-carboxylic acid (15) (180 mg, 715 μmol), EDC·HCl (374 mg, 1.95 mmol) and DMF (16 ml) and stirred for 1 d at 20 °C. The reaction mixture was adjusted to pH 2 with HCl (2 n) and extracted with EtOAc (4 × 20 ml). The combined organic phases were washed with water (3 × 20 ml), brine (20 ml), dried (MgSO4) and concentrated under reduced pressure to give a brown solid. This material was adsorbed on silica gel and subjected to column chromatography (pentane/EtOAc = 3:1) to yield 16 (196 mg, 43 % over two steps) as green solid. Rf = 0.57 (pentane/ EtOAc = 2:1); UV/VIS (CH3CN): λmax (lg ε) = 209 (4.758), 271 (4.470), 315 (4.465), 363 nm (4.524); IR (KBr): ν̃ = 3461 (NH), 2951, 1711 (C=O), 1624, 1527, 1459, 1408, 1309, 1107, 837, 747 cm–1; 1H NMR (300 MHz, CDCl3): δ = 0.09 (s, 9 H, Si(CH3)3), 1.13–1.19 (m, 2 H, CH2SiMe3), 3.42 (dd, J = 10.8, 10.2 Hz, 1 H, 10-Hb), 3.90–4.05 (m, 11 H, 3 × OCH3, 1-H, 10-Ha), 4.43–4.48 (m, 2 H, CH2OC=O), 4.56 (dd, J = 11.1, 8.7 Hz, 1 H, 2-Hb), 4.71 (dd, J = 11.1, 1.8 Hz, 1 H, 2-Ha), 5.25–5.31 (m, 2 H, CH2Ph), 6.85 (s, 1 H, 4′-H), 6.96 (d, J = 2.4 Hz, 1 H, 3′-H), 7.31–7.55 (m, 5 H, 5 × Ph-H), 7.62 (d, J = 9.0 Hz, 1 H, 9-H), 8.07 (dd, J = 9.0, 1.8 Hz, 1 H, 8-H), 8.21 (s, 1 H, 4-H), 9.03 (d, J = 1.8 Hz, 1 H, 6-H), 9.75 ppm (d, J = 2.4 Hz, 1 H, indole-NH); 13C NMR (75 MHz, CDCl3): δ = –1.47 (Si(CH3)3), 17.26 (CH2SiMe3), 42.72 (C-1), 45.88 (C-10), 55.11 (C-2), 56.11, 61.00, 61.36 (3 × OCH3), 63.18 (CH2OC=O), 70.30 (CH2Ph), 97.54 (C-4′), 98.87 (C-4), 106.75 (C-3′), 115.94, 123.45, 125.65, 131.71, 144.30 (C-3a, C-5a, C-7, C-9a, C-9b), 121.99 (C-9), 122.48, 125.61, 129.48, 138.72, 140.55 (C-2′, C-3a′, C-6′, C-7′, C-7a′), 126.47 (C-6), 127.12 (C-8), 127.44, 127.97, 128.49 (5 × Ph-C), 136.22 (Ph-Ci), 150.08 (C-5′), 156.65 (C-5), 160.51 (C=O), 166.62 ppm (C(O)OTMSE); MS (ESI): m/z (%) = 723 (100) [M + Na]+, 1423 (75) [2M + Na]+, 699 (100) [M – H]–; HRMS: calcd for C38H41ClN2O7Si: 701.2444 [M + H]+; found: 701.2441.
(1R/S)-1-Chloromethyl-5-hydroxy-3-(5,6,7-trimethoxyindole-2-carbonyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (3b): A magnetically stirred solution of benzyl ether 16 (152 mg, 217 μmol) in 3:1 THF/MeOH (6 ml) was treated with 10 % Pd/C (62 mg) and dropwise with NH4HCO2 (572 μl of a 25 % solution in water, 2.26 mmol) and stirring continued for 75 min at 20 °C. The reaction mixture was filtered through a pad of Celite® and it was thoroughly washed with MeOH and THF. The filtrate was dried (MgSO4) and concentrated under reduced pressure to give 3b (133 mg, quant.) as yellow solid which was used for the next reaction without further purification. Rf = 0.25 (pentane/ EtOAc = 2:1); 1H NMR (300 MHz, [D6]DMSO): δ = 0.09 (s, 9 H, Si(CH3)3), 1.12–1.17 (m, 2 H, CH2SiMe3), 3.81–3.88 (m, 7 H, 2 × OCH3, 10-Hb), 3.95 (s, 3 H, OCH3), 4.02 (dd, J = 11.1, 3.1 Hz, 1 H, 10-Ha), 4.20 (mc, 1 H, 1-H), 4.41–4.50 (m, 3 H, CH2OC=O, 2-Hb), 4.74 (dd, J = 11.1, 9.1 Hz, 1 H, 2-Ha), 6.97 (s, 1 H, 4′-H), 7.07 (d, J = 2.0 Hz, 1 H, 3′-H), 7.91–7.98 (m, 3 H, 4-H, 8-H, 9-H), 8.82 (d, J = 1.0 Hz, 1 H, 6-H), 10.83 (s, 1 H, OH), 11.42 ppm (d, J = 2.0 Hz, 1 H, indole-NH); 13C NMR (75 MHz, [D6]DMSO): δ = –1.45 (Si(CH3)3), 16.87 (CH2SiMe3), 40.72 (C-1), 47.36 (C-10), 55.09 (C-2), 55.93, 60.84, 61.00 (3 × OCH3), 62.56 (CH2OC=O), 98.07 (C-4′), 100.82 (C-4), 106.31 (C-3′), 115.12, 123.06, 125.45, 131.92, 144.76 (C-3a, C-5a, C-7, C-9a, C-9b), 120.95, 123.12, 130.69, 139.00, 139.94 (C-2′, C-3a′, C-6′, C-7′, C-7a′), 123.94 (C-9), 125.89 (C-8), 126.02 (C-6), 149.19 (C-5′), 155.57 (C-5), 160.39 (C=OTMI), 165.89 ppm (C(O)OTMSE); MS (ESI): m/z (%) = 633 (100) [M + Na]+, 1243 (29) [2M + Na]+, 573 (100) [M – H – HCl]–, 609 (22) [M – H]–; HRMS: calcd for C31H35ClN2O7Si: 611.1975 [M + H]+; found: 611.1975.
(1R/S)-5-(2-Benzyloxycarbonyl-ethylcarbamoyloxy)-1-chloromethyl-3-(5,6,7-trimethoxyindo-le-2-carbonyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (17): A magnetically stirred solution of phenol 3b (198 μmol, 121 mg) in CH2Cl2 (15 ml) at 0 °C was treated dropwise with 13 (203 μl, 203 mg; 990 μmol) and then triethylamine (139 μl, 100 mg, 990 μmol). The reaction mixture was warmed to 20 °C and stirred for a further 16 h. The ensuing solution was cooled to 0 °C, adjusted to pH 2 with HCl (2 n) and extracted with EtOAc (4 × 10 ml). The combined organic phases were washed with brine (10 ml), dried (MgSO4) and concentrated under reduced pressure to give a yellow solid. This material was adsorbed on silica gel and subjected to column chromatography (pentane/EtOAc = 1:1) to afford carbamate 17 (135 mg, 83 %) as yellow solid. Rf = 0.29 (pentane/EtOAc = 2:1); 1H NMR (300 MHz, [D6]DMSO, 100 °C): δ = 0.10 (s, 9 H, Si(CH3)3), 1.13–1.19 (m, 2 H, CH2SiMe3), 2.71 (t, J = 7.1 Hz, 2 H, 2″-H2), 3.47–3.53 (m, 2 H, 1″-H2), 3.85 (s, 6 H, 2 × OCH3), 3.98 (dd, J = 11.2, 6.8 Hz, 1 H, 10-Hb), 4.00 (s, 3 H, OCH3), 4.09 (dd, J = 11.2, 3.4 Hz, 1 H, 10-Ha), 4.39 (mc, 1 H, 1-H), 4.45–4.50 (m, 2 H, CH2OC=O), 4.58 (dd, J = 11.1, 2.6 Hz, 1 H, 2-Hb), 4.80 (dd, J = 11.1, 9.2 Hz, 1 H, 2-Ha), 5.17 (s, 2 H, CH2Ph), 6.99 (s, 1 H, 4′-H), 7.09 (d, J = 2.2 Hz, 1 H, 3′-H), 7.30–7.41 (m, 5 H, 5 × Ph-H), 8.04 (dd, J = 8.7, 1.4 Hz, 1 H, 8-H), 8.09 (d, J = 8.7 Hz, 1 H, 9-H), 8.24 (s, 1 H, 4-H), 8.62 (d, J = 1.4 Hz, 1 H, 6-H), 11.02 ppm (brs, 1 H, indole-NH); 13C NMR (75 MHz, [D6]DMSO, 100 °C): δ = –0.95 (Si(CH3)3), 17.61 (CH2SiMe3), 34.62/34.68 (C-2″), 37.58/37.60 (C-1″), 41.76 (C-1), 47.79/47.84 (C-10), 55.62 (C-2), 56.99 (OCH3), 61.38 (2 × OCH3), 63.36 (CH2OC=O), 66.15 (CH2Ph), 99.48 (C-4′), 107.05 (C-3′), 111.34 (C-4), 122.42, 124.17, 126.73, 132.19, 144.43 (C-3a, C-5a, C-7, C-9a, C-9b), 123.78, 126.36, 131.09, 139.56, 140.95 (C-2′, C-3a′, C-6′, C-7′, C-7a′), 124.29 (C-9), 125.41 (C-6), 126.78 (C-8), 128.23 (2 × Ph-C), 128.35 (Ph-Cp), 128.81 (2 × Ph-C), 136.72 (Ph-Ci), 149.15 (C-5′), 150.13 (OC(O)N), 156.16/1546.18 (C-5), 161.23 (C=OTMI), 166.19 (C(O)OTMSE), 171.23 ppm (C(O)OBn); MS (ESI): m/z (%) = 611 (100) [M – C(O)NH-β-Ala-OBn + H]+, 838 (18) [M + Na]+, 1653 (14) [2M + Na]+, 573 (100) [M – C(O)NH-β-Ala-OBn – HCl]–, 609 (55) [M – C(O)NH-β-Ala-OBn – H]–.
(1R/S)-5-(2-Carboxy-ethylcarbamoyloxy)-1-chloromethyl-3-(5,6,7-trimethoxyindole-2-carbo-nyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (18): A magnetically stirred solution of benzyl ester 17 (203 mg, 249 μmol) in 3:1 THF/MeOH (10 ml) was treated with 10 % Pd/C (83 mg) and dropwise with NH4HCO2 (653 μl of a 25 % solution in water, 2.59 mmol). Stirring was continued for 25 min (TLC-monitoring necessary as the alanyl residue is easily cleaved off) at 20 °C. The reaction mixture was filtered through a pad of Celite®, which was thoroughly washed with MeOH and CH2Cl2. The combined filtrates were concentrated under reduced pressure, the residue dissolved in CH2Cl2, dried (MgSO4) and concentrated under reduced pressure again to give a yellow oil. This material was adsorbed on silica gel and subjected to column chromatography (CH2Cl2/ MeOH = 20:1) to afford acid 18 (163 mg, 90 %) as pale yellow solid. Rf = 0.44 (CH2Cl2/MeOH = 10:1); 1H NMR (300 MHz, CDCl3): δ = 0.06 (s, 9 H, Si(CH3)3), 1.12 (mc, 2 H, CH2SiMe3), 2.74 (mc, 2 H, 2″-H2), 3.41 (mc, 1 H, 10-Hb), 3.65 (mc, 2 H, 1″-H2), 3.83–4.07 (m, 11 H, 1-H, 10-Ha, 3 × OCH3), 4.42 (mc, 2 H, CH2OC=O), 4.57–4.64 (m, 1 H, 2-Hb), 4.71–4.74 (m, 1 H, 2-Ha), 6.39 (brs, 1 H, NH), 6.87 (brs, 1 H, 4′-H), 6.99 (brs, 1 H, 3′-H), 7.62 (mc, 1 H, 9-H), 8.01 (mc, 1 H, 8-H), 8.41 (brs, 1 H, 4-H), 8.56 (brs, 1 H, 6-H), 9.99 ppm (brs, 1 H, indole-NH); 13C NMR (75 MHz, CDCl3): δ = –1.46 (Si(CH3)3), 17.34 (CH2SiMe3), 34.17 (C-2″), 36.99 (C-1″), 43.01 (C-1), 45.75 (C-10), 55.17 (C-2), 56.22, 61.31, 61.49 (3 × OCH3), 63.65 (CH2OC=O), 97.78 (C-4′), 107.11 (C-3′), 111.78 (C-4), 121.31, 124.21, 126.85, 131.42, 143.39 (C-3a, C-5a, C-7, C-9a, C-9b), 122.50 (C-9), 123.56, 125.86, 129.29, 138.68, 140.83 (C-2′, C-3a′, C-6′, C-7′, C-7a′), 125.93 (C-6), 126.52 (C-8), 149.00 (C-5′), 150.10 (OC(O)N), 154.39 (C-5), 160.58 (C=OTMI), 166.61 (C(O)OTMSE), 171.50 ppm (C(O)OH); MS (ESI): m/z (%) = 748 (90) [M + Na]+, 1473 (100) [2M + Na]+, 573 (96) [M – C(O)NH-β-Ala-OH – HCl]–, 1449 (100) [2M – H]–.
(1R/S)-1-Chloromethyl-5-[2-(N-succinimidyloxycarbonyl)-ethyl-carbamoyloxy]-3-(5,6,7-tri-methoxyindole-2-carbonyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (19): A magnetically stirred solution of acid 18 (110 mg, 152 μmol) and N-hydroxysuccinimide (26.0 mg, 228 μmol) in 1:1 THF/CH2Cl2 (10 ml) at 0 °C was treated with EDC·HCl (44.0 mg, 228 μmol). The reaction mixture was warmed to 20 °C and stirring continued for 15 h. The ensuing solution was then adjusted to pH 2 with HCl (2 n) and extracted with EtOAc (4 × 10 ml). The combined organic phases were washed with brine (10 ml), dried (MgSO4) and concentrated under reduced pressure to afford 19 as yellow solid which was used for the next reaction without further purification. Rf = 0.83 (CH2Cl2/MeOH = 10:1); MS (ESI): m/z (%) = 845 (90) [M + Na]+, 1667 (100) [2M + Na]+, 573 (100) [M – C(O)NH-β-Ala-OSu – HCl]–.
(1R/S)-1-Chloromethyl-5-(l-phenylalaninamidyl-l-aspartyl-l-methionyl-l-tryptophyl-β-alanyl-carbonyloxy)-3-(5,6,7-trimethoxyindole-2-carbonyl)-2,3-dihydro-1H-benz[e]indole-7-carboxylic acid [2-(trimethylsilyl)-ethyl] ester (2): A magnetically stirred solution of tetragastrin (20)[27] (39.4 mg, 66.0 μmol) in water (1 ml) was treated with NEtiPr2 (11.5 μl, 8.50 mg, 66.0 μmol) and dropwise with a solution of crude 19 (49 mg, 60 μmol) in DMF (3.5 ml). Stirring was continued for 7 h at 20 °C. The ensuing mixture was adjusted to pH 2 with HCl (2 n) and extracted with EtOAc (5 × 5 ml). The combined organic phases were washed with water (5 ml) and brine (5 ml), treated with toluene (5 ml) and concentrated under reduced pressure. The resulting yellow solid was adsorbed on silica gel and subjected to column chromatography (CH2Cl2/MeOH = 15:1 + 0.5 % HOAc → 10:1 + 0.5 % HOAc) to give 2 (45 mg, 57 % over two steps, 83 % based on recovery, 1:1 mixture of both diastereomeres) as light yellow solid. Further purification was achieved by HPLC. Rf = 0.27 (CH2Cl2/MeOH = 10:1 + 0.5 % HOAc); 1H NMR (600 MHz, [D7]DMF): δ = 0.10/0.12 (2 × s, 9 H, Si(CH3)3), 1.20 (mc, 2 H, CH2SiMe3), 1.98–2.07 (m, 5 H, 2c-H2, SCH3), 2.37–2.66 (m, 6 H, 2b-H2, 3c-H2, 1e-H2), 2.98–3.06 (m, 3 H, 2a-Hb, 1f-H2), 3.17 (dd, J = 14.8, 8.4 Hz, 1 H, 2d-Hb), 3.21–3.24 (m, 1 H, 2a-Ha), 3.28–3.33 (m, 1 H, 2d-Ha), 3.88 (s, 3 H, OCH3), 3.90 (s, 3 H, OCH3), 3.95 (dd, J = 11.1, 7.9 Hz, 1 H, 10-Hb), 4.03 (s, 3 H, OCH3), 4.12 (dd, J = 11.1, 3.3 Hz, 1 H, 10-Ha), 4.30 (mc, 1 H, 1-H), 4.42–4.47 (m, 1 H, 1c-H), 4.50 (mc, 2 H, CH2OC=O), 4.58 (mc, 1 H, 1a-H), 4.64 (mc, 1 H, 1b-H), 4.67 (dd, J = 10.7, 2.2 Hz, 1 H, 2-Hb), 4.73 (mc, 1 H, 1d-H), 4.85 (dd, J = 10.7, 9.1 Hz, 1 H, 2-Ha), 6.57 (s, 1 H, NH), 6.97 (mc, 1 H, 5d′-H), 7.05 (s, 1 H, 4′-H), 7.06 (mc, 1 H, 6d′-H), 7.17 (mc, 1 H, 1 × Ph-H), 7.19 (mc, 2 H, 3′-H, 2d′-H), 7.24–7.32 (m, 4 H, 4 × Ph-H), 7.37/7.39 (2 × d, J = 8.0 Hz, 1 H, 7d′-H), 7.57/7.63 (2 × d, J = 7.9 Hz, 1 H, 4d′-H), 7.73 (brs, 1 H, NH), 7.83 (brs, 1 H, NH), 8.02 (d, J = 8.8 Hz, 1 H, 9-H), 8.05 (dd, J = 8.8, 1.6 Hz, 1 H, 8-H), 8.11 (brs, 1 H, 4-H), 8.18–8.44 (m, 4 H, NH), 8.99 (d, J = 1.6 Hz, 1 H, 6-H), 10.82/10.87 (2 × s, 1 H, indole-NHTrp), 11.34 ppm (s, 1 H, indole-NHTMI); 13C NMR (150 MHz, [D7]DMF): δ = –1.46/–1.42 (Si(CH3)3), 15.01 (SCH3), 17.67/17.76 (CH2SiMe3), 28.12 (C-2d), 30.79 (C-3c), 31.88 (C-2c), 32.09 (C-1e), 35.02 (C-1f), 38.16 (C-2a), 39.40 (C-2b), 42.21 (C-1), 47.94 (C-10), 51.56 (C-1b), 53.65 (C-1c), 55.07 (C-1d), 55.27 (C-1a), 56.03 (C-2), 56.46/56.49, 61.36, 61.43 (3 × OCH3), 63.38 (CH2OC=O), 98.89 (C-4′), 101.95 (C-4), 107.19 (C-3′), 110.62 (C-3d′), 111.93 (C-7d′), 116.06, 124.35, 126.61, 131.78, 145.99 (C-3a, C-5a, C-7, C-9a, C-9b), 118.89 (C-5d′), 118.95 (C-4d′), 121.46/121.51 (C-6d′), 122.17, 129.86, 133.05, 139.97, 141.16 (C-2′, C-3a′, C-6′, C-7′, C-7a′), 123.75 (C-9), 124.41 (C-2d′), 126.85, 126.91 (C-6, C-8, Ph-Cp), 128.51 (C-3ad′), 128.78 (2 × Ph-C), 129.83 (2 × Ph-C), 137.33 (C-7ad′), 139.04 (Ph-Ci), 150.54 (C-5′), 152.52 (OC(O)N), 156.90 (C-5), 161.36 (C=OTMI), 162.20 (C=OAla), 166.96 (C(O)OTMSE), 170.71, 171.25, 172.27, 172.33 (4 × C=O), 184.62 ppm (C(O)OH); MS (ESI): m/z (%) = 633 (100) [M – C(O)NH-pentapeptide + H + Na]+, 573 (100) [M – C(O)NH-pentapeptide – Cl]–; HRMS: calcd for C64H74ClN9O15SSi: 1304.4556 [M + H]+; found: 1304.4558; HPLC (preparative): column: Kromasil 100 C18; eluent: 85 % MeOH, 15 % H2O + 0.05 % TFA; flow: 12 mL/min; Rt: 31–40 min.
Cell culture: Human bronchial carcinoma cells of line A549 (ATCC CCL 185) were kindly provided by the Institut für Zellbiologie, Universität Essen, and human pancreatic carcinoma cells Mia PaCa-2 by the Universitätsklinikum Göttingen, Abteilung Hämatologie und Onkologie. Cell lines were maintained as exponentially growing cultures at 37 °C and 7.5% CO2 in air in culture medium (DMEM (Biochrom) supplemented with 10 % fetal calf serum, 44 mm NaHCO3 (Biochrom) and 4 mm l-Glutamine (Invitrogen)).
In vitro cytotoxicity assays: Cells of line A549 or MIA PaCa-2 were seeded in duplicates in 6 multiwell plates at concentrations of 102, 103 and 104 cells per well. After cells were allowed to adhear, cells were washed in a serum-free incubation medium (Ultraculture medium, Lonza). Incubation with compounds 2 and 3b was then performed in Ultraculture medium at various concentrations for 24 h. All substances were used as freshly prepared solutions in DMSO (Merck) diluted with incubation medium to a final concentration of DMSO of 1% in the wells. After exposure the test substance was removed and cells were washed with fresh medium. Cultivation in normal growth medium was done for 10 days. The medium was removed, the clones were dried and stained with Löffler's methylene blue (Merck) and then counted macroscopically.
The IC50 values are based on the relative colony-forming rate, which was determined according to the following formula: relative colony-forming rate [%] = 100 × (number of clones counted after exposure) / (number of clones counted in the control).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. F. M. thanks the Studienstiftung des deutschen Volkes (German National Academic Foundation) for a Ph.D. scholarship. B. K. is grateful to the Deutsche Telekom Foundation for a Ph.D. scholarship.
References and Notes
- Bagshawe, KD. Antibody directed enzymes revive anti-cancer prodrugs concept. Br. J. Cancer 1987, 56, 531–532, Reviews:. [Google Scholar] Tietze, LF; Feuerstein, T. Enzyme and Proton-Activated Prodrugs for a Selective Cancer Therapy. Curr. Pharm. Des. 2003, 9, 2155–2175. [Google Scholar] Tietze, LF; Feuerstein, T. Highly Selective Compounds for the Antibody-Directed Enzyme Prodrug Therapy of Cancer. Aust. J. Chem. 2003, 56, 841–854. [Google Scholar] Jung, M. Antibody directed enzyme prodrug therapy (ADEPT) and related approaches for anticancer therapy. Mini-Rev. Med. Chem. 2001, 1, 399–407. [Google Scholar] Syrigos, KN; Epenetos, AA. Antibody directed enzyme prodrug therapy (ADEPT): a review of the experimental and clinical considerations. Anticancer Res. 1999, 19, 605–613. [Google Scholar] Springer, CJ; Niculescu-Duvaz, I. Antibody-directed enzyme prodrug therapy (ADEPT): a review. Adv. Drug Deliv. Rev. 1997, 26, 151–172. [Google Scholar]
- Tietze, L; Major, F; Schuberth, I; Spiegl, DA; Krewer, B; Maksimenka, K; Bringmann, G; Magull, J. Selective Treatment of Cancer: Synthesis, Biological Evaluation and Structural Elucidation of Novel Analogues of the Antibiotic CC-1065 and the Duocarmycins. Chem. Eur. J. 2007, 13, 4396–4409. [Google Scholar] Tietze, LF; Major, F; Schuberth, I. Antitumor Agents: Development of Highly Potent Glycosidic Duocarmycin Analogues for Selective Cancer Therapy. Angew. Chem. 2006, 118, 6724–6727. [Google Scholar] Angew. Chem., Int. Ed. 2006, 45, 6574–6577. Tietze, LF; Krewer, B; Frauendorf, H; Major, F; Schuberth, I. Investigation of Reactivity and Selectivity of DNA-Alkylating Duocarmycin Analogues by High-Resolution Mass Spectrometry. Angew. Chem. 2006, 118, 6720–6724. [Google Scholar] Angew. Chem., Int. Ed. 2006, 45, 6570–6574.
- Ganesh, T. Improved biochemical strategies for targeted delivery of taxoids. Bioorg. Med. Chem. 2007, 15, 3597–3623. [Google Scholar] Devalapally, H; Navath, RS; Yenamandra, V; Akkinepally, RR; Devarakonda, RK. Directed Enzyme Prodrug Therapy/Prodrug MonoTherapy. Arch. Pharm. Res. 2007, 30, 723–732. [Google Scholar] Alaoui, AE; Saha, N; Schmidt, F; Monneret, C; Florent, J-C. New Taxol®(paclitaxel) prodrugs designed for ADEPT and PMT strategies in cancer chemotherapy. Bioorg. Med. Chem. 2006, 14, 5012–5019. [Google Scholar] Kratz, F; Warnecke, A; Schmid, B; Chung, D-E; Gitzel, M. Prodrugs of anthracyclines in cancer chemotherapy. Curr. Med. Chem. 2006, 13, 477–523. [Google Scholar] Schmidt, F; Monneret, C. Prodrug Monotherapy: synthesis and biological evaluation of an etoposide glucuronide-prodrug. Bioorg. Med. Chem. 2003, 11, 2277–2283. [Google Scholar] Prijovich, ZM; Chen, BM; Leu, Y-L; Chern, J-W; Roffler, SR. Anti-tumour activity and toxicity of the new prodrug 9-aminocamptothecin glucuronide (9 ACG) in mice. Br. J. Cancer. 2002, 86, 1634–1638. [Google Scholar] Denny, WA. Prodrug strategies in cancer therapy. Eur. J. Med. Chem. 2001, 36, 577–595. [Google Scholar] Bosslet, K; Straub, R; Blumrich, M; Czech, J; Gerken, M; Sperker, B; Kroemer, HK; Gesson, J-P; Koch, M; Monneret, C. Elucidation of the Mechanism Enabling Tumor Selective Prodrug Monotherapy. Cancer Res. 1998, 58, 1195–1201. [Google Scholar] Bosslet, K; Czech, J; Hoffmann, D. Tumor Targeting 1995, 1, 45–50.
- Reubi, J; Mäcke, HR; Krenning, EP. Candidates for peptide receptor radiotherapy today and in the future. J. Nucl. Med. 2005, 46, 67S–75S. [Google Scholar] Dyba, M; Tarasova, NI; Michejda, CJ. Small Molecule Toxins Targeting Tumor Receptors. Curr. Pharm. Des. 2004, 10, 2311–2334. [Google Scholar] Reubi, JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocrine Reviews 2003, 24, 389–427. [Google Scholar] Reubi, JC; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. 2003, 30, 781–793. [Google Scholar] Langer, M; Beck-Sickinger, AG. Peptides as Carrier for Tumor Diagnosis and Treatment. Curr. Med. Chem. 2001, 1, 71–93. [Google Scholar] Breeman, WAP; de Jong, M; Kwekkeboom, DJ; Valkema, R; Bakker, WH; Kooij, PPM; Visser, TJ; Krenning, EP. Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives. Eur. J. Nucl. Med. 2001, 28, 1421–1429. [Google Scholar] Heppeler, A; Froidevaux, S; Eberle, AN; Maecke, HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr. Med. Chem. 2000, 7, 971–994. [Google Scholar]
- Nock, BA; Maina, T; Béhé, M; Nikolopoulou, A; Gotthardt, M; Schmitt, JS; Behr, TM; Macke, HR. CCK-2/Gastrin Receptor–Targeted Tumor Imaging with99mTc-Labeled Minigastrin Analogs. J. Nucl. Med. 2005, 46, 1727–1736. [Google Scholar] Béhé, M; Behr, TM. Cholecystokinin-B (CCK-B)/gastrin receptor targeting peptides for staging and therapy of medullary thyroid cancer and other CCK-B receptor expressing malignancies. Biopolymers (Peptide Science) 2002, 66, 399–418. [Google Scholar] Behr, TM; Jenner, N; Béhé, M; Angerstein, C; Gratz, S; Raue, F; Becker, W. Radiolabeled Peptides for Targeting Cholecystokinin-B/Gastrin Receptor-Expressing Tumors. J. Nucl. Med. 1999, 40, 1029–1044. [Google Scholar] Behr, TM; Jenner, N; Radetzky, S; Béhé, M; Gratz, S; Yücekent, S; Raue, F; Becker, W. Targeting of cholecystokinin-B/gastrin receptors in vivo: preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur. J. Nucl. Med. 1998, 25, 424–430. [Google Scholar]
- Schmidt, BF; Hernandez, L; Rouzer, C; Czerwinski, G; Chmurny, G; Michejda, CJ. Peptide-Linked 1,3-Dialkyl-3-acyltriazenes:Gastrin Receptor Directed Antineoplastic Alkylating Agents. J. Med. Chem. 1994, 37, 3812–3818. [Google Scholar]
- Czerwinski, G; Tarasova, NI; Michejda, CJ. Cytotoxic agents directed to peptide hormone receptors: Defining the requirements for a successful drug. Proc. Natl. Acad. Sci. USA 1998, 95, 11520–11525. [Google Scholar]
- Buchholz, S; Keller, G; Schally, AV; Halmos, G; Hohla, F; Heinrich, E; Koester, F; Baker, B; Engel, JB. Therapy of ovarian cancers with targeted cytotoxic analogs of bombesin, somatostatin, and luteinizing hormone-releasing hormone and their combinations. Proc. Natl. Acad. Sci. USA 2006, 103, 10403–10407. [Google Scholar] Nagy, A; Schally, AV. Minireview. Targeting of Cytotoxic Luteinizing Hormone-Releasing Hormone Analogs to Breast, Ovarian, Endometrial, and Prostate Cancers. Biol. Reprod. 2005, 73, 851–859. [Google Scholar] Nagy, A; Schally, AV; Armatis, P; Szepesházi, K; Halmos, G; Kovács, M; Zarandi, M; Groot, K; Miyazaki, M; Jungwirth, A; Horvath, J. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500-1000 times more potent. Proc. Natl. Acad. Sci. USA 1996, 93, 7269–7273. [Google Scholar]
- Engel, JB; Keller, G; Schally, AV; Halmos, G; Hammann, B; Nagy, A. Effective Inhibition of Experimental Human Ovarian Cancers with a Targeted Cytotoxic Bombesin Analogue AN-215. Clin. Cancer Res. 2005, 11, 2408–2415. [Google Scholar] Nagy, A; Armatis, P; Cai, RZ; Szepesházi, K; Halmos, G; Schally, AV. Design, synthesis, and in vitro evaluation of cytotoxic analogs of bombesin-like peptides containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin . Proc. Natl. Acad. Sci. USA 1997, 94, 652–656. [Google Scholar]
- Szepesházi, K; Schally, AV; Halmos, G; Armatis, P; Hebert, F; Sun, B; Feil, A; Kiaris, H; Nagy, A. Targeted Cytotoxic Somatostatin Analogue AN-238 Inhibits Somatostatin Receptor-positive Experimental Colon Cancers Independently of Their p53 Status. Cancer Res. 2002, 62, 781–788. [Google Scholar] Nagy, A; Schally, AV; Halmos, G; Armatis, P; Cai, RZ; Csernus, V; Kovács, M; Koppán, M; Szepesházi, K; Kahán, Z. Synthesis and biological evaluation of cytotoxic analogs of somatostatin containing doxorubicin or its intensely potent derivative, 2-pyrrolino-doxorubicin. Proc. Natl. Acad. Sci. USA 1998, 95, 1794–1799. [Google Scholar]
- Langer, M; Kratz, F; Rothen-Rutishauser, B; Wunderli-Allenspach, H; Beck-Sickinger, AG. Novel Peptide Conjugates for Tumor-Specific Chemotherapy. J. Med. Chem. 2001, 44, 1341–1348. [Google Scholar]
- Tietze, LF; Panknin, O; Major, F; Krewer, B. Synthesis of a Novel Pentagastrin-Drug Conjugate for a Targeted Tumor Therapy. Chem. Eur. J. 2008, 14, 2811–2818. [Google Scholar]
- Boger, DL; Johnson, DS. CC-1065 and the Duocarmycins: Understanding their Biological Function through Mechanistic Studies. Angew. Chem. 1996, 108, 1542–1580. [Google Scholar] Angew. Chem. Int. Ed. Engl. 1996, 35, 1438–1474. Ichimura, M; Ogawa, T; Katsumata, S; Takahashi, K; Takahashi, I; Nakano, H. Duocarmycins, new antitumor antibiotics produced by streptomyces; producing organisms and improved production. J. Antibiot. 1991, 44, 1045–1053. [Google Scholar] Ichimura, M; Ogawa, T; Takahashi, K; Kobayashi, E; Kawamoto, I; Yasuzawa, T; Takahashi, I; Nakano, H. Duocarmycin, a new antitumor antibiotic from streptomyces sp. J. Antibiot. 1990, 43, 1037–1038. [Google Scholar]
- Boger, DL; Bollinger, B; Hertzog, DL; Johnson, DS; Cai, H; Mésini, P; Garbaccio, RM; Jin, Q; Kitos, PA. Reversed and Sandwiched Analogs of Duocarmycin SA: Establishment of the Origin of the Sequence-Selective Alkylation of DNA and New Insights into the Source of Catalysis. J. Am. Chem. Soc. 1997, 119, 4987–4998. [Google Scholar] Boger, DL; Johnson, DS. CC-1065 and the Duocarmycins: Understanding their Biological Function through Mechanistic Studies. Angew. Chem. 1996, 108, 1542–1580. [Google Scholar] Angew. Chem. Int. Ed. Engl. 1996, 35, 1438–1474. Hurley, LH; Needham-VanDevanter, DR. Covalent Binding of Antitumor Antibiotics in the Minor Groove of DNA. Mechanism of Action of CC-1065 and the Pyrrolo(1,4) benzodiazepines. Acc. Chem. Res. 1986, 19, 230–237. [Google Scholar]
- Tietze, LF; Haunert, F; Feuerstein, T; Herzig, T. A Concise and Efficient Synthesis of seco-Duocarmycin SA. Eur. J. Org. Chem. 2003, 3, 562–566. [Google Scholar] Baird, R; Winstein, S. Neighboring Carbon and Hydrogen. Li. Dienones from Ar1[UNK]-3 Participation. Isolation and Behavior of Spiro(2,5)octa-1,4-diene-3-one. J. Am. Chem. Soc. 1963, 85, 567–578. [Google Scholar]
- Morley, JS; Tracy, HJ; Gregory, RA. Structure–Function Relationships in the Active C-Terminal Tetrapeptide Sequence of Gastrin. Nature 1965, 1356–1359. [Google Scholar] Tracy, HJ; Gregory, RA. The antral Hormone Gastrin: Physiological Properties of a Series of Synthetic Peptides structurally related to Gastrin I. Nature 1964, 204, 935–938. [Google Scholar]
- Alberts, SR; Erlichman, C; Reid, JM; Sloan, JA; Ames, MM; Richardson, RL; Goldberg, RM. Phase I study of the duocarmycin semisynthetic derivative KW-2189 given daily for five days every six weeks. Clin. Cancer Res. 1998, 4, 2111–2117. [Google Scholar] Nagamura, S; Kobayashi, E; Gomi, K; Saito, H. Studies on the active metabolite (DU-86) of KW-2189, a novel derivative of duocarmycin. Bioorg. Med. Chem. Lett. 1996, 6, 2147–2150. [Google Scholar] Nagamura, S; Kobayashi, E; Gomi, K; Saito, H. Synthesis and antitumor activity of duocarmycin derivatives: A-ring pyrrole analogues of duocarmycin B2. Bioorg. Med. Chem. 1996, 4, 1379–1391. [Google Scholar] Nagamura, S; Asai, A; Kanda, Y; Kobayashi, E; Gomi, K; Saito, H. Synthesis and Antitumor Activity of Duocarmycin Derivatives: Modification of Segment A of Duocarmycin B2. Chem. Pharm. Bull. 1996, 44, 1723–1730. [Google Scholar] Yasuzawa, T; Muroi, K; Ichimura, M; Takahashi, I; Ogawa, T; Takahashi, K; Sano, H; Saitoh, Y. Duocarmycins, Potent Antitumor Antibiotics Produced by Streptomyces sp. Structures and Chemistry. Chem. Pharm. Bull. 1995, 43, 378–391. [Google Scholar] Asai, A; Nagamura, S; Saito, S. A Novel Property of Duocarmycin and Its Analogs for Covalent Reaction with DNA. J. Am. Chem. Soc. 1994, 116, 4171–4177. [Google Scholar]
- Ray, S; Chaturvedi, D. Application of organic carbamates in drug design. Part 1: Anti-cancer agents-recent reports. Drugs Fut. 2004, 29, 343–357. [Google Scholar]
- Kaiser, N-F; Hallberg, A; Larhed, M. In situ Generation of Carbon Monoxide from Solid Molybdenum Hexacarbonyl. A Convenient and Fast Route to Palladium-Catalyzed Carbonylation Reactions. J. Comb. Chem. 2002, 4, 109–111. [Google Scholar]
- Boger, DL; Han, N; Tarby, CM; Boyce, CW; Cai, H; Jin, Q; Kitos, PA. Synthesis, Chemical Properties, and Preliminary Evaluation of Substituted CBI Analogs of CC-1065 and the Duocarmycins Incorporating the 7-Cyano-1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one Alkylation Subunit: Hammett Quantitation of the Magnitude of Electronic Effects on Functional Reactivity. J. Org. Chem. 1996, 61, 4894–4912. [Google Scholar]
- Boger, DL; Brunette, SR; Garbaccio, RM. Synthesis and Evaluation of a Series of C3-Substituted CBI Analogues of CC-1065 and the Duocarmycins. J. Org. Chem. 2001, 66, 5163–5173. [Google Scholar] Boger, DL; McKie, JA; Cai, H; Cacciari, B; Baraldi, PG. Synthesis and Properties of Substituted CBI Analogs of CC-1065 and the Duocarmycins Incorporating the 7-Methoxy-1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one (MCBI) Alkylation Subunit: Magnitude of Electronic Effects on the Functional Reactivity. J. Org. Chem. 1996, 61, 1710–1729. [Google Scholar]
- Boger, DL; Boyce, CW; Garbaccio, RM; Searcey, M. Synthesis of CC-1065 and duocarmycin analogs via intramolecular aryl radical cyclization of a tethered vinyl chloride. Tetrahedron Lett. 1998, 39, 2227–2230. [Google Scholar] Patel, VF; Andis, SL; Enkema, JK; Johnson, DA; Kennedy, JH; Mohamadi, F; Schultz, RM; Soose, DJ; Spees, MM. Total Synthesis of Seco (+)- and ent-(−)-Oxaduocarmycin SA: Construction of the (Chloromethyl)indoline Alkylating Subunit by a Novel Intramolecular Aryl Radical Cyclization onto a Vinyl Chloride. J. Org. Chem. 1997, 62, 8868–8874. [Google Scholar] Boger, DL; McKie, JA. An Efficient Synthesis of 1,2,9,9a-Tetrahydrocyclopropa[c]benz[e]indol-4-one CBI: An Enhanced and Simplified Analog of the CC-1065 and Duocarmycin Alkylation Subunits. J. Org. Chem. 1995, 60, 1271–1275. [Google Scholar]
- Studer, A; Amrein, S. Silylated Cyclohexadienes: New Alternatives to Tributyltin Hydride in Free Radical Chemistry. Angew. Chem. 2000, 112, 3196–3198. [Google Scholar] Angew. Chem. Int. Ed. 2000, 39, 3080–3082. Chatgilialoglu, C. Organosilanes as radical-based reducing agents in synthesis. Acc. Chem. Res. 1992, 25, 188. [Google Scholar]
- Belshaw, PJ; Mzengeza, S; Lajoie, GA. Chlorotrimethylsilane Mediated Formation of ω-Allyl Esters of Aspartic And Glutamic Acids. Synth. Commun. 1990, 20, 3157–3160. [Google Scholar]
- Mehta, A; Jaouhari, R; Benson, TJ; Douglas, KT. Improved efficiency and selectivity in peptide synthesis: use of triethylsilane as a carbocation scavenger in deprotection of t-butyl esters and t-butoxycarbonyl-protected sites. Tetrahedron Lett. 1992, 33, 5441–5444. [Google Scholar]
- Ram, S; Spicer, LD. Rapid debenzylation of N-benzylamino derivatives to amino-derivatives using ammonium formate as catalytic hydrogen transfer agent. Tetrahedron Lett. 1987, 28, 515–516. [Google Scholar] Bieg, T; Szeja, W. Removal of-Benzyl Protective Groups by Catalytic Transfer Hydrogenation. Synthesis 1985, 76–77. [Google Scholar]
- Tetragastrin (20) was purchased from Bachem.