Abstract
Dietary polyphenols represent a wide variety of compounds that occur in fruits, vegetables, wine, tea, extra virgin olive oil, chocolate and other cocoa products. They are mostly derivatives and/or isomers of flavones, isoflavones, flavonols, catechins and phenolic acids, and possess diverse biological properties such as antioxidant, antiapoptosis, anti-aging, anticarcinogen, anti-inflammation, anti-atherosclerosis, cardiovascular protection, improvement of the endothelial function, as well as inhibition of angiogenesis and cell proliferation activity. Most of these biological actions have been attributed to their intrinsic reducing capabilities. They may also offer indirect protection by activating endogenous defense systems and by modulating cellular signaling processes such as nuclear factor-kappa B (NF-κB) activation, activator protein-1(AP-1) DNA binding, glutathione biosynthesis, phosphoinositide 3 (PI3)-kinase/protein kinase B (Akt) pathway, mitogen-activated protein kinase (MAPK) proteins [extracellular signal-regulated protein kinase (ERK), c-jun N-terminal kinase (JNK) and P38 ] activation, and the translocation into the nucleus of nuclear factor erythroid 2 related factor 2 (Nrf2). This paper covers the most recent literature on the subject, and describes the biological mechanisms of action and protective effects of dietary polyphenols.
1. Introduction
Oxidative stress results in oxidative alteration of biological macromolecules such as lipids, proteins and nucleic acids. It is considered to play a pivotal role in the pathogenesis of aging and degenerative diseases [1–3]. In order to cope with an excess of free radicals produced upon oxidative stress, human bodies have developed sophisticated mechanisms for maintaining redox homeostasis. These protective mechanisms include scavenging or detoxification of reactive oxygen species (ROS), blocking ROS production, sequestration of transition metals, as well as enzymatic and nonenzymatic antioxidant defenses produced in the body, that is, endogenous [4,5], and others supplied with the diet, namely, exogenous ones. Among them, dietary polyphenols have been widely studied for their strong antioxidant capacities and other properties by which cell functions are regulated [6,7].
Dietary polyphenols represent a group of secondary metabolites which widely occur in fruits, vegetables, wine, tea, extra virgin olive oil, chocolate and other cocoa products. They are mostly derivatives, and/or isomers of flavones, isoflavones, flavonols, catechins, and phenolic acids. Dietary polyphenols exhibit many biologically significant functions, such as protection against oxidative stress, and degenerative diseases. Experimental data indicate that most of these biological actions can be attributed to their intrinsic antioxidant capabilities. Dietary polyphenols may offer an indirect protection by activating endogenous defense systems and by modulating cellular signaling processes such as NF-κB activation, AP-1 DNA binding, glutathione biosynthesis, PI3-kinase/Akt pathway, MAPK proteins (ERK, JNK and P38) activation, and the translocation into the nucleus of Nrf2 [8–10].
2. Classification and occurrence of dietary polyphenols
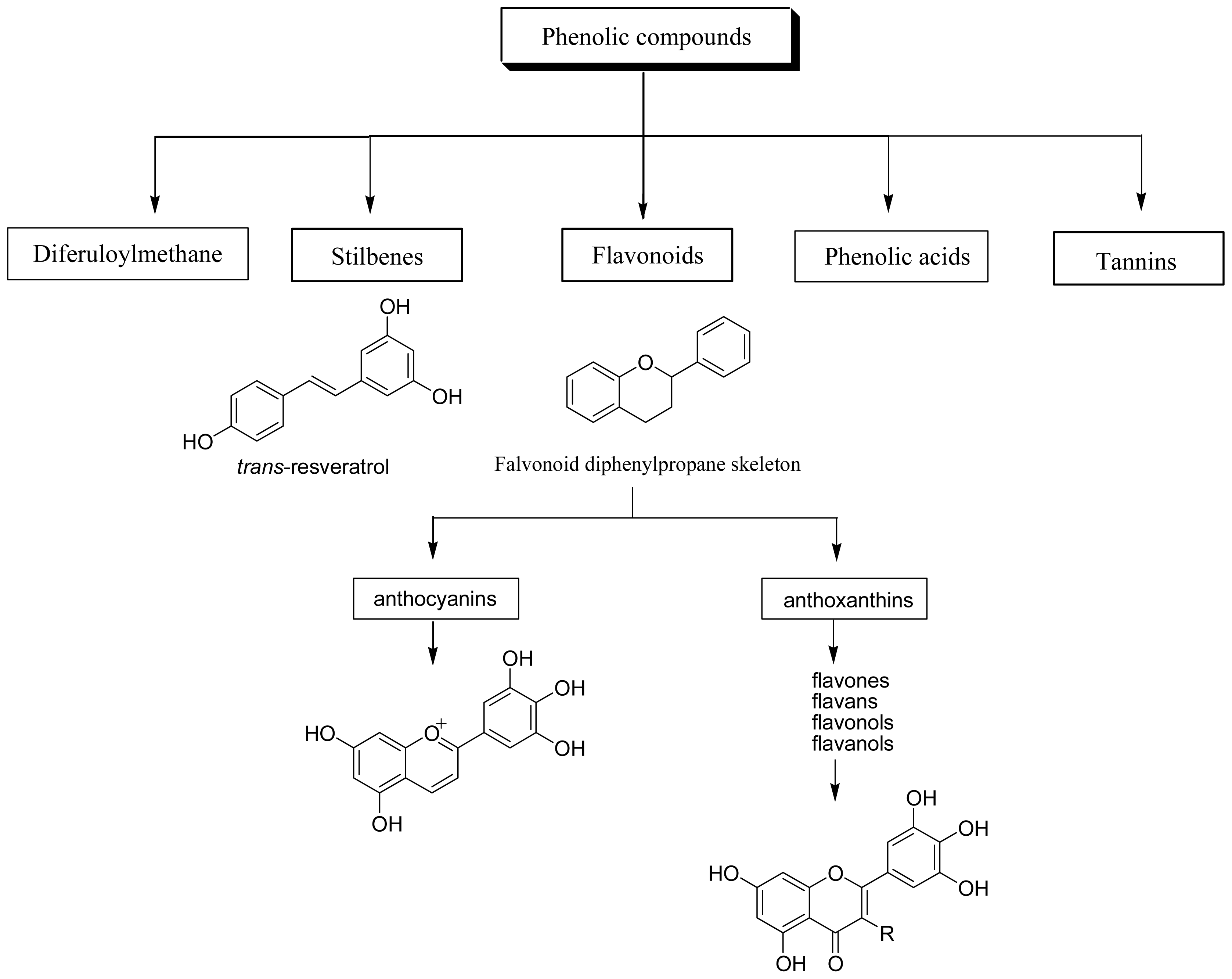
Dietary polyphenols are the most abundant antioxidants in human diets. With over 8,000 structural variants, they are secondary metabolites of plants and denote many substances with aromatic ring(s) bearing one or more hydroxyl moieties. They are subdivided into groups (Figure 1) by the number of phenolic rings and of the structural elements that link these rings [11]: (1) The phenolic acids with the subclasses derived from hydroxybenzoic acids such as gallic acid and from hydroxycinnamic acid, containing caffeic, ferulic, and coumaric acid; (2) the large flavonoid subclass, which includes the flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols; (3) the stilbenes; and (4) the lignans and the polymeric lignins.
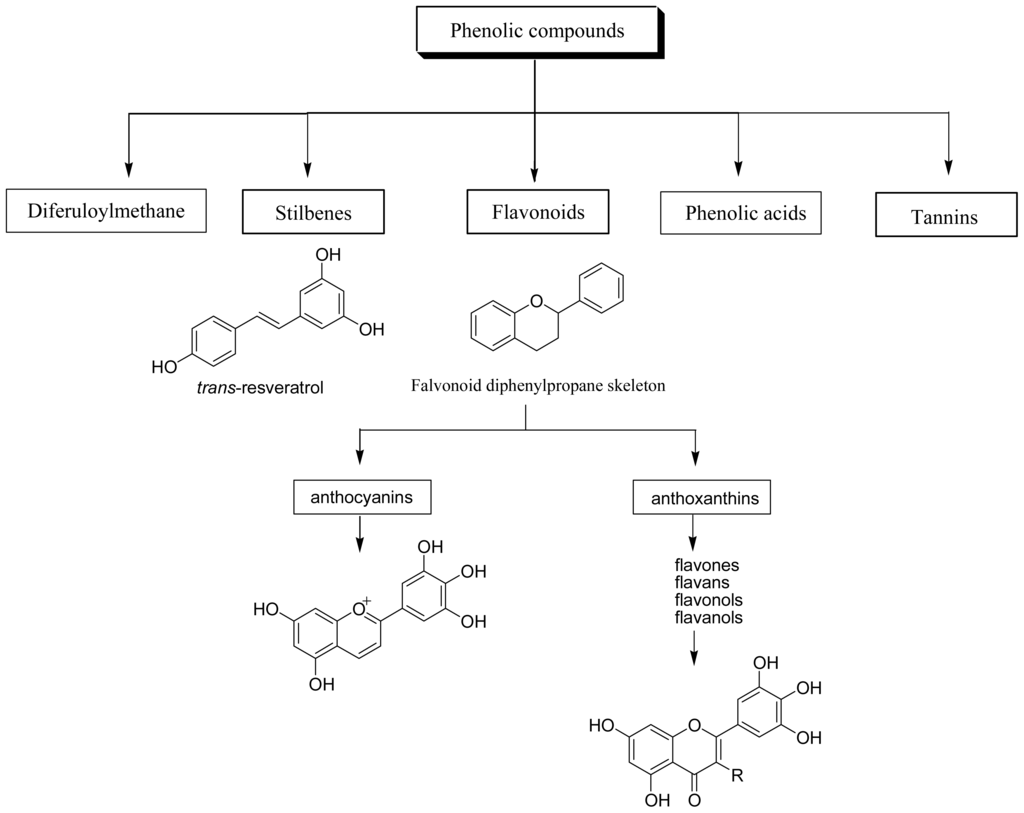
Figure 1.
Classification of dietary polyphenols.
The main dietary sources of polyphenols include some common fruits, vegetables and beverages. Phenolic acids account for about one third of the total intake and flavonoids account for the remaining two thirds. The most abundant flavonoids in the diet are flavanols (catechins plus proanthocyanidins), anthocyanins and their oxidation products. The main polyphenol dietary sources are fruit and beverages (fruit juice, wine, tea, coffee, chocolate and beer) and, to a lesser extent vegetables, dry legumes and cereals. Most of dietary polyphenols and their sources in our diets were shown in Table 1.

Table 1.
Classification and sources of dietary polyphenols
2.1 Phenolic acids
A major class within the phenolic compounds is the hydroxycinnamic acids, which are widely distributed in plant kingdom. The major hydroxycinnamic acid is caffeic acid, which occurs in foods mainly as an ester with quinic acid called chlorogenic acid (5-caffeoylquinic acid). Chlorogenic acid and caffeic acid are antioxidants in vitro and they might inhibit the formation of mutagenic and carcinogenic N-nitroso compounds for the inhibitory effect on the N-nitrosation reaction in vitro.
2.2 Flavonoids
Flavonoids are the most abundant polyphenols in human diets, and are mainly divided into: (a) anthocyanins, glycosylated derivative of anthocyanidin, present in colorful flowers and fruits; (b) anthoxanthins, a group of colorless compounds further divided in several categories, including flavones, flavans, flavonols, flavanols, isoflavones, and their glycosides. Flavonols are mainly represented by myricetin, fisetin, quercetin and kaempferol.
2.3 Stilbenes
Stibenes are structurally characterized by the presence of a 1,2-diphenylethylene nucleus with hydroxyls substitued on the aromatic rings, and exist in the form of monomers or oligomers. The best known compound is trans-resveratrol, possessing a trihydroxystilbene skelelton.
2.4 Tannins
Tannins are a group of water-soluble polyphenols having molecular weights from 500 to 3,000 which are subdivided into condensed and hydrolisable tannins, and commonly found complexed with alkaloids, polysaccharides and proteins, particularly the latter. On the basis of structural characteristics there are two groups, gallotannins and ellagitannins of hydrolysable tannins.
2.5 Diferuloylmethanes
Diferuloylmethanes are a small group of phenolic compounds with two aromatic rings substitued with hydroxyls, and linked by aliphatic chain containing carbonyl groups. There are also some other polyphenols such as hydroxytyrosol, a simple polyphenol presenting in olive fruits and olive oil [12,13].
3. Bioactivities of dietary polyphenols
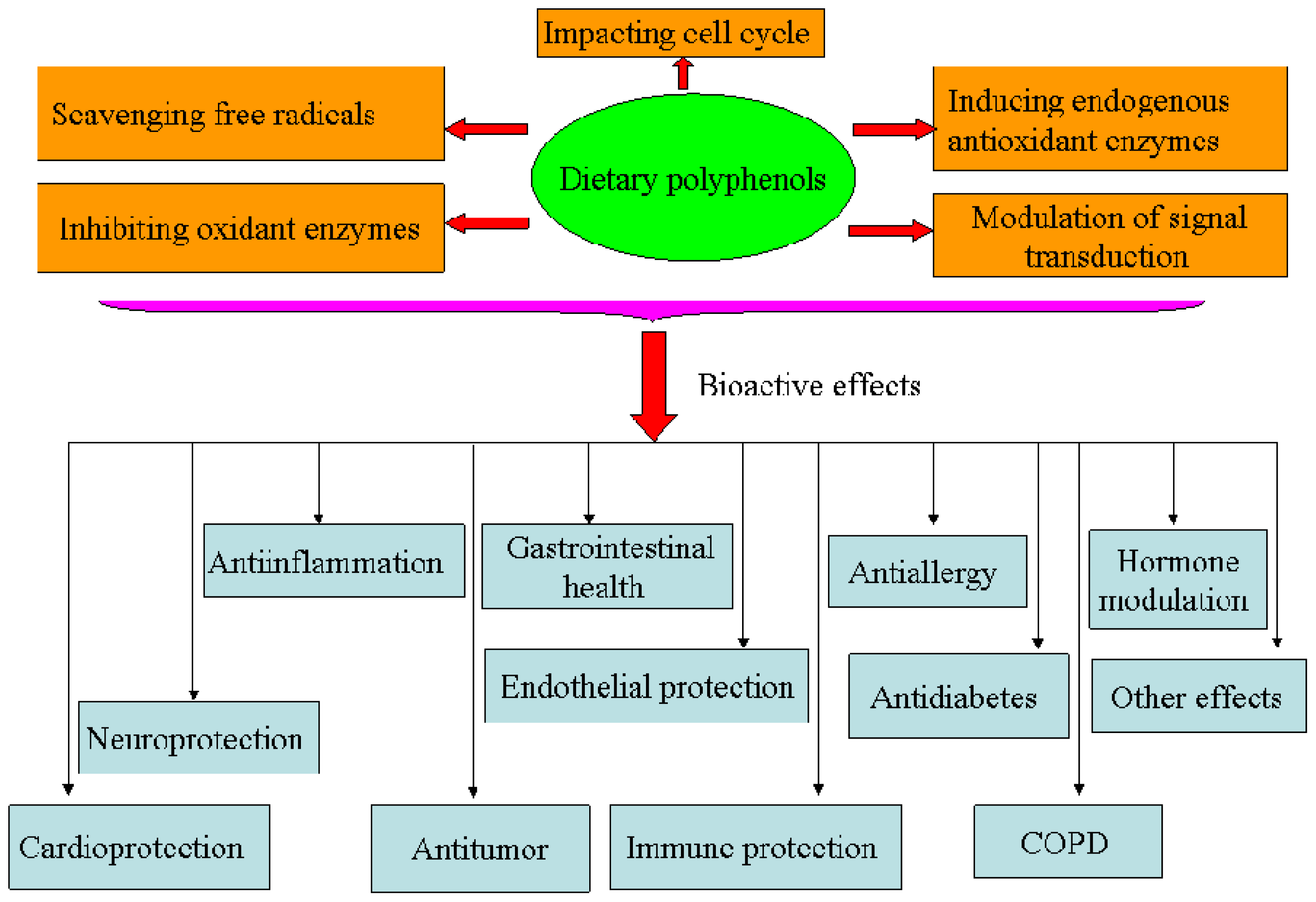
Oxidative stress is considered to play a pivotal role in the pathogenesis of aging and several degenerative diseases, such as atherosclerosis, cardiovascular disease, type II diabetes and cancer [1–3]. In order to cope with an excess of free radicals produced upon oxidative stress, humans have developed endogenous and exogenous mechanisms in order to maintain redox homeostasis. Among these, dietary polyphenols have been largely studied for their strong antioxidant capacities and other properties by which cell activities are regulated (Figures 2 and 3).
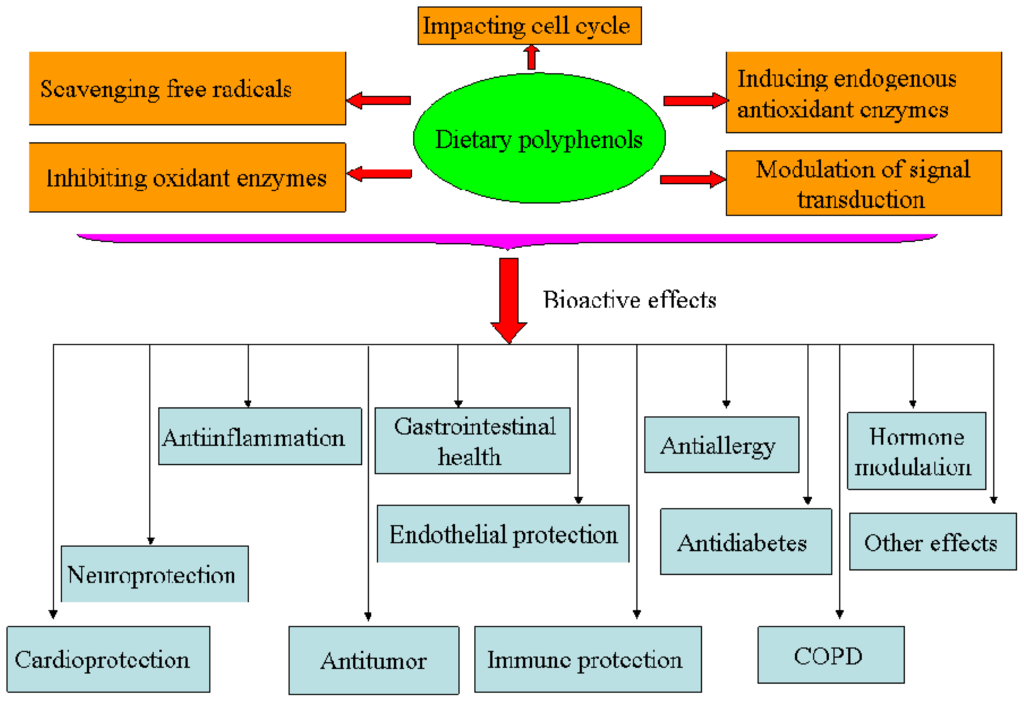
Figure 2.
Bioactivities of dietary polyphenols.
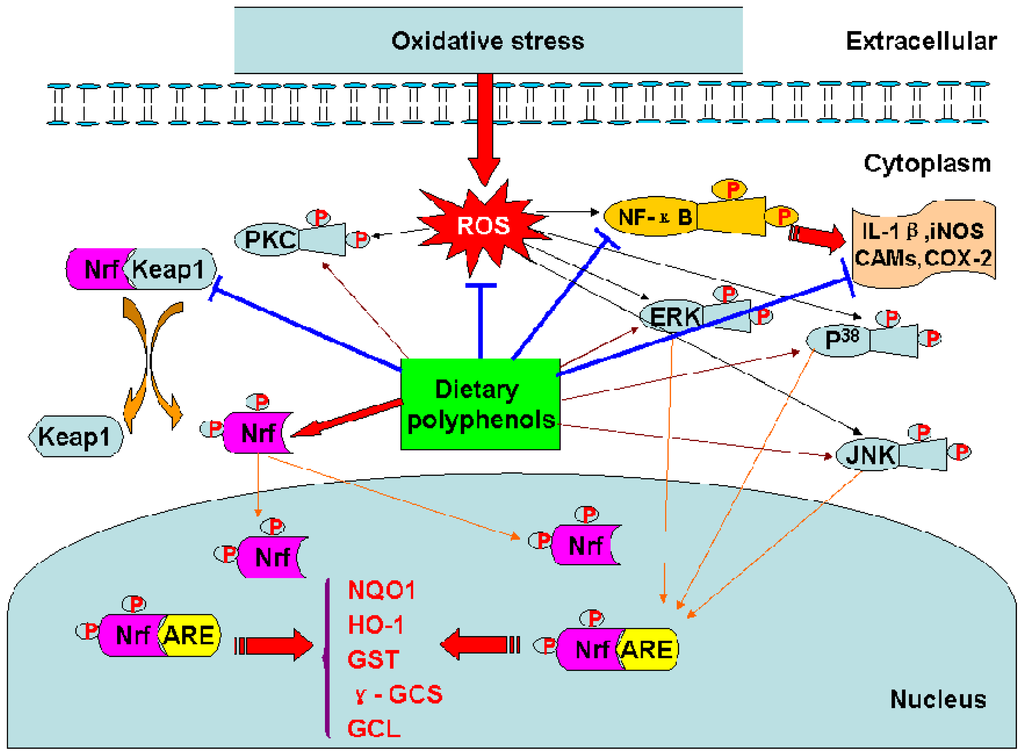
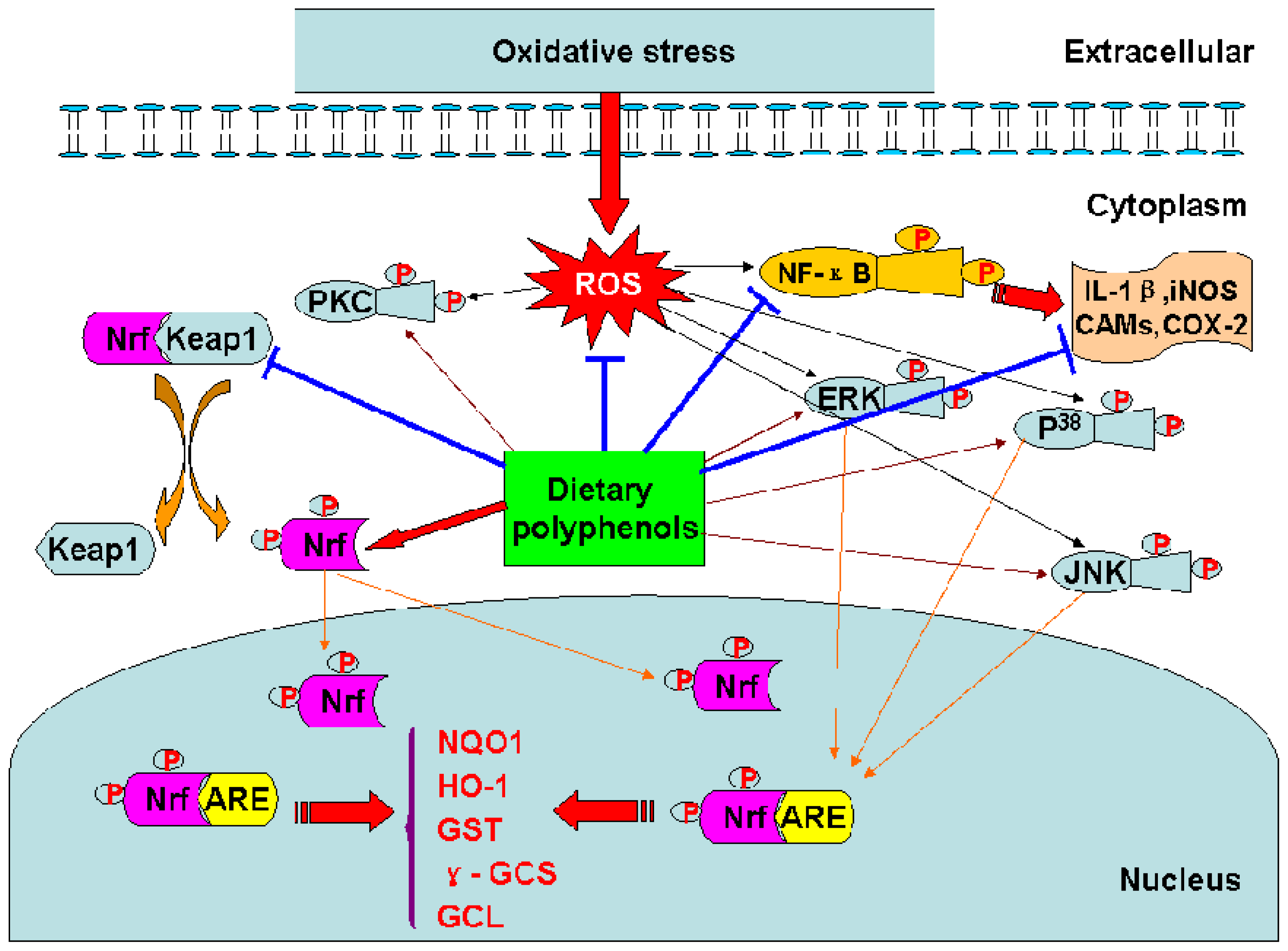
Figure 3.
Mechanisms of the biological effects of dietary polyphenols.
3.1 Antioxidant and free radical scavenging properties
In order to combat and neutralize the deleterious effects of ROS, various antioxidant strategies have evolved either by increasing the endogenous antioxidant enzyme defenses or by enhancing the non-enzymatic defenses through dietary or pharmacological means (Table 2). Dietary polyphenols have been reported to possess potent antioxidant activity by endogenous and exogenous mechanisms.

Table 2.
Antioxidant and free radical scavenging properties of dietary polyphenols.
Dihydrocaffeic acid was able to scavenge free radicals (superoxide anion, hydroxyl and peroxyl radicals) in human EA.hy926 endothelial cells [42]. Curcumin and quercetin increased several antioxidant enzyme activities such as glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT) or glutathione reductase (GR) in vivo and in vitro[8,9,44], and activated endogenous defense systems in vitro[40,45]. Hydroxytyrosol could increase CAT and SOD activities in rats fed a cholesterol-rich diet [35].
The transcription factor Nrf2 regulates the basal and inducible expression of numerous detoxifying and antioxidant genes. The Nrf2–Kelch-like ECH-associated protein 1 (Keap1)-ARE system is now recognized as one of the major cellular defence mechanisms against oxidative and xenobiotic stresses [46]. (−)-Epigallochatechin gallate (EGCG) and (−)-epichatechin gallate (ECG) induced ARE-mediated gene expression through the activation of MAPK proteins (ERK, JNK and p38) in HepG2-ARE-C8 cell [10]. Tanigawa et al. reported that quercetin-induced ARE activity involves upregulation of Nrf2 through the regulation of both transcription and posttranscription sites and repression of Keap1 by affecting the posttranscription site in HepG2 cells [48]. Curcumin could increase the expression of glutathione S-transferase P1 (GSTP1) by activing ARE and Nrf2 in HepG2 cells [40].
3.2. Anti-atherosclerosis and cardioprotection
Studies have shown that some of dietary polyphenols exerted anti-atherosclerosis and cardioprotection (Table 3). Oleuropein inhibited the oxidation of low density lipoprotein (LDL) in vitro[61]. Quercetin decreased lipid peroxidation, upregulated the expression of serum high density lipoprotein (HDL)-associated paraoxonase 1(PON-1) in the HuH7 human hepatoma cell line [66], inhibited oxidized LDL (oxLDL)-triggered apoptosis, and increased intracellular glutathione (GSH) downregulation in COS-1 cells [68].

Table 3.
Anti-atherosclerosis and cardioprotection of dietary polyphenols.
Proanthocyanidin could significantly reduce cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and c-Jun in Male Sprague Dawley rats [74]. Furthermore, proanthocyanidin could regulate the levels of CD36 mRNA and protein in oxLDL treated peripheral blood mononuclear cells [73]. Resveratrol showed that in vitro it could decrease the expression of vascular cell adhesion molecule-1 (VCAM-1) [64], cyclooxygenase-2 (COX-2) [55], and matrix metalloproteinase-9 (MMP-9) mRNA [56] through suppression of activation of nuclear factor AP-1 [55]. Hydroxytyrosol could not only lower serum total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C), but also slow the lipid peroxidation process in rats fed a cholesterol-rich diet [35].
3.3 Neuroprotective effects on anti-aging and neurodegenerative diseases
Recently, there has been considerable interest in the neuroprotective effects of dietary polyphenols (Table 4), especially in the context of their modes of action as antioxidants [6]. Resveratrol had an impact on cognitive deficits by activating the phosphorylation of protein kinase C (PKC), secreting transthyretin to prevent Aβ aggregation in cultured rat hippocampal cells [77], and stimulating AMP kinase activity in Neuro2a cells and primary neurons [75]. EGCG stimulated the deacetylase activity of recombinant silent information regulator two ortholog 1 (SIRT1) protein in human HT29 cells [80]. Curcumin could disrupt existing plaques and restore distorted neurites in an Alzheimer mouse model [84]. They had been considered as therapeutic agents for altering brain aging processes, and as possible neuroprotective agents in progressive neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases.

Table 4.
Neuroprotective effects of dietary polyphenols.
3.4 Anti-inflammatory properties
Oxidative stress induced inflammation is mediated by the activation of NF-kB and AP-1. It affects a wide variety of cellular signaling processes leading to generation of inflammatory mediators and chromatin remodeling [95,96]. The latter allows expression of pro-inflammatory genes such as interleukin-1beta (IL-1β), IL-8, tumor necrotic factor alpha (TNF-a), and inducible nitric oxide synthase (iNOS). The undesired effects of oxidative stress have been found to be controlled by the antioxidant and/or anti-inflammatory effects of dietary polyphenols such as curcumin and resveratrol in vivo and in vitro[88–90,95,97] (Table 5). Resveratrol inhibited pro-inflammatory gene expression via inhibition of inhibitory κB (IκB), thus inhibiting NF-κB transactivation, as well as restoring transrepressive pathways through the activation of histone deacetylases in RAW 264.7 cells [89].

Table 5.
Anti-inflammatory effects of dietary polyphenols.
On the other hand, to counter the effects of oxidative stress, the cells also concomitantly express protective antioxidants such as glutamate cysteine ligase (GCL), manganese superoxide dismutase (MnSOD), and heme oxygenase-1(HO-1). In addition, expression of these antioxidant genes via modulation of MAPK-ARE-Nrf2 pathway is upregulated by EGCG and ECG in HepG2-ARE-C8 cell [10]. Apigenin, luteolin and quercetin had also been reported to inhibit inflammatory responses by downregulating the expression of iNOS and adhesion molecules in NR8383 macrophages and human endothelial cells [91–93].
3.5 Antimutagenic/anticarcinogenic properties
Dietary polyphenols could modulate diverse biochemical processes involved in carcinogenesis (Table 6). Curcumin exerted antitumor activities by inhibition of cellular proliferation and angiogenesis, blockade of tumor cell cycle progression, and induction of programmed cell death in vivo and in vitro [109,110]. Cellular signaling cascades mediated by NF-κB or AP-1 acted as a centerplay in regulating many of aforementioned biochemical processes [102,110].

Table 6.
Antimutagenic/anticarcinogenic properties of dietary polyphenols.
Resveratrol could block the activation of MAPKs and AP-1 in the skin of mice [102]. Consumption of berries and red fruits rich in polyphenols contributed to the reduction of cancer through many mechanisms such as in vitro inhibiting human cytochrome P450-dependent monooxygenases 1A1 (CYP1A1) activities [26], blocking the epidermal growth factor receptor (EGFR) tyrosine kinase activity [107], and decreasing protein kinase CKII activity [103].
3.6 Maintenance of gastrointestinal health and effects on digestive enzymes
It had been reported that digestive enzymes such as lipase, α-amylase, and α-glucosidase, were inhibited by proanthocyanidins and tannins in young chicks, which decreased the digestibility of protein, starch and lipid [119, 120]. Resveratrol could inhibit pancreatic bile salt-dependent lipase (BSDL) activity, expression and secretion in the rat pancreatic AR4-2J cells [121]. Cyanidin-3α-O-rhamnoside and quercetin-3α-O-rhamnoside could inhibit α-glucosidase and advanced glycation end product (AGE) formation in vitro[123]. The inhibition of digestive enzymes by dietary polyphenols may represent an under-reported mechanism for delivering some of the health benefits attributed to a diet rich in fruit and vegetables.
3.7 Modulation of signal transduction pathways
Dietary polyphenols may not merely exert their diverse biological effects as free radical scavengers, but may also modulate cellular signaling processes by affecting signal transduction pathways [122] (Table 7). Studies have been reported that curcumin could in vitro modulate NF-κB activation [124], AP-1 DNA binding [126], signal transducer and activator of transcription-3 (STAT3) phosphorylation [118]. Resveratrol exerted protection in vitro through PI3-kinase/Akt pathway, MAPK proteins (ERK, JNK and P38) activation [58], and the translocation into the nucleus of Nrf2 [132]. Resveratrol could also upregulate the expressions of GCL, MnSOD, and HO-1 against oxidative stress via MAPK-ARE-Nrf2 pathway in PC12 cells [132].

Table 7.
Effects of dietary polyphenols on signal transduction pathways.
3.8 Improvement of endothelium functions
Several studies have indicated that red wine polyphenolic compounds (RWPCs) were able to inhibit proliferation and migration of vascular cells (Table 8). RWPCs induced nitric oxide (NO)-mediated endothelium-dependent relaxations in isolated arteries. The activation of endothelial NO synthase (eNOS) was due to two distinct mechanisms: (a) an increase in [Ca2+] i and (b) a phosphorylation of eNOS by the PI3-kinase/Akt pathway [137]. In addition, RWPCs caused endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxations of isolated arteries consecutively to a localized and controlled formation of superoxide anions leading to the activation of the PI3-kinase/Akt pathway [136]. RWPCs also increased endothelial prostacyclin release and inhibited the synthesis and the effects of endothelin-1 in endothelial cell [139,141].

Table 8.
Protective effects of dietary polyphenols on endothelial cells and blood vessels
RWPCs could prevent matrix metalloproteinases-2 (MMP-2) activation and vascular endothelial growth factor (VEGF) expression in vascular smooth muscle cells (VSMCs) [133,134]. All these mechanisms might contribute to explain the vasodilatory, vasoprotective and anti-hypertensive effects of polyphenols in vivo.
Cyanidin-3-glucoside (Cy3G) and EGCG could enhance vascular eNOS activity and improve vascular endothelial function in bovine vascular endothelial cells [142]. Catechins had anti-angiogenic effects by reducing the vascularization on the chicken chorioallantoic membrane (CAM) [145].
3.9 Protective effect on immune cell functions
Dietary polyphenols appear to have a protective effect on immune cell functions. Alvarez et al. showed that leukocyte functions were improved in prematurely aging mice after five weeks of diet supplementation with polyphenol-rich cereals [149]. They could increase macrophage chemotaxis, phagocytosis, microbicidal activity, and natural killer function, and increase lymphoproliferation and IL-2 release in response to concanavalin A and lipopolysaccharide.
Curcumin could prevent tumor-induced T cell apoptosis by downregulating Bax level and augmenting Bcl-2 expression and restore cytokine-dependent Jak-3/Stat-5a signaling pathway in T cells of tumor bearer [150]. Caffeic acid, ellagic acid, and ferulic acid could inhibit apoptosis through the Bcl-2 independent mechanism in normal human peripheral blood mononuclear cells [116]. Thus, regular intake of these compounds will protect and improve quality of life.
3.10 Antiallergic activity
The incidence of type I allergic disorders have been increasing worldwide, particularly, the hypersensitivity to food. Akiyama and his coworkers reported that the apple condensed tannins intake would inhibit the development of the oral sensitization, and the inhibition could correlate with the rise in the population of TCRγδ-T cells in the intestinal intraepithelial lymphocytes [151]. Moreover, the apple condensed tannins could inhibit the release of histamine from rat basophilic leukemia (RBL-2H3) cells stimulated by the antigen-stimulation and from rat peritoneal mast cells stimulated by compound 48/80. They also inhibited hyaluronidase activity and increase in intracellular free calcium concentration in RBL-2H3 cells stimulated with the antigen [152].
3.11 Antidiabetic effects
Johnston and coworkers demonstrated that glucose uptake into cells under sodium-dependent conditions was inhibited by flavonoid glycosides and non-glycosylated polyphenols in polarised Caco-2 intestinal cells [154]. Under sodium-free conditions, aglycones and non-glycosylated polyphenols inhibited glucose uptake whereas glycosides and phenolic acids were ineffective. These data suggest that aglycones inhibit facilitated glucose uptake whereas glycosides inhibit the active transport of glucose. The non-glycosylated dietary polyphenols appeared to exert their effects via steric hindrance, while EGCG, ECG and (−)-epigallochatechin were effective against both transporters.
More recently, Koboyashi et al. have shown that the green tea polyphenols EGCG and ECG also inhibited glucose transport, possibly by sodium-dependent glucose transporter 1 (SGLT1) inhibition in the rabbit small intestine [155]. Song et al have presented evidence for quercetin-mediated inhibition of the facilitated diffusion glucose transporter 2 (GLUT2) in Chinese hamster ovary cells [156].
Anthocyanins inhibited α-glucosidase activity and reduced blood glucose levels after starch-rich meals. This is a proven clinical therapy for controlling type II diabetes [158] (Table 9).

Table 9.
Antidiabetic activity of dietary polyphenols.
3.12 Regulation of cell cycle progression
It was demonstrated that resveratrol and proanthocyanidins could regulate cell cycle progression by upregulating p21 expression, G1 phase arrest and downregulating cyclin D1/D2–Cdk6 in vitro [163–165, 170] (Table 10).

Table 10.
Regulate cell cycle progression of dietary polyphenols.
3.13 Modulation of hormonal effects and contraceptive activity
Some studies showed that dietary polyphenols could modulate the level of hormone. Resveratrol could exert mixed estrogen agonist/antagonist activities in mammary tumor models. It could affect the expression of 17β-estradiol-responsive progesterone receptor (PR) and presnelin 2 proteins in vitro and in vivo [159]. Bhat et al. showed that resveratrol exhibited antiestrogenic properties and inhibited the levels and activity of PR by downregulating α (1)-integrin expression in human endometrial adenocarcinoma cells [160].
Otake and his coworkers demonstrated that quercetin and resveratrol potently reduced estrogen sulfotransferase (EST) activity and inhibited sulfation of 17β-estradiol in normal human mammary epithelial cells [161]. Both of the compounds potently inhibited recombinant human EST. In fact, they could serve as substrates for EST. Gossypol, a polyphenolic compound from cotton seed, had contraceptive activity and could inhibit 11β-hydroxysteroid dehydrogenase and cause hypokalemia in some men [162].
3.14 Effect in the treatment of chronic obstructive pulmonary disease (COPD)
Since a variety of oxidants and free radicals are implicated in the pathogenesis of COPD, it is possible that therapeutic administration of multiple antioxidants will be effective in the treatment of COPD. Various approaches to enhance lung antioxidant capacity and clinical trials of dietary polyphenols in COPD are discussed. Resveratrol, EGCG, and quercetin could inhibit inflammatory gene expression by controling NF-κB activation and regulate GSH biosynthesis and chromatin remodel in human airway epithelial A549 cells [171,172]. Curcumin could decrease protein/mRNA expressions of pulmonary type I collagen (Col-I) and TGF-β1 in rats [173].
3.15 Other bioactive effects
It has been demonstrated that dietary polyphenols have other bioactive effects (Table 11), such as antibacterial activity of Gnemonol B and gnetin E [174], anti-HIV effect of proanthocyanidins [176], hepatoprotective ability of a novel proanthocyanidins IH636 [178], and angiogenesis effect of proanthocyanidins [177].

Table 11.
Other bioactive effects of dietary polyphenols.
4. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary polyphenols
Dietary polyphenols have beneficial antioxidant, anti-inflammatory and anticancer effects. However, at higher doses or under certain conditions these compounds may exert toxic prooxidant activities [181]. Galati et al.[182] have observed that dietary polyphenols with phenol rings were metabolized by peroxidase to form prooxidant phenoxyl radicals which, in some cases were sufficiently reactive to cooxidize GSH or NADH accompanied by extensive oxygen uptake and reactive oxygen species formation. Polyphenols with catechol rings also cooxidized ascorbate, likely mediated by semiquinone radicals. Incubation of hepatocytes with dietary polyphenols containing phenol rings was found to partially oxidize hepatocyte GSH to GSSG while polyphenols with a catechol ring were found to deplete GSH through formation of GSH conjugates.
Dietary polyphenols with phenol rings also oxidized human erythrocyte oxyhemoglobin and caused erythrocyte hemolysis more readily than polyphenols with catechol rings. It is concluded that polyphenols containing a phenol ring are generally more prooxidant than polyphenols containing a catechol ring. Subsequent studies revealed that [183] B-ring catechol-type flavonoids showed swift formation of their two electron oxidized quinone type metabolites, even upon their one electron oxidation by peroxidases. Enzymatic and/or chemical (auto) oxidation of the flavonoid generates the flavonoid semiquinone radical, which may be scavenged by GSH, thereby regenerating the flavonoid and generating the thiyl radical of glutathione. This thiyl radical may react with GSH to generate a disulfide radical anion which rapidly reduces molecular oxygen to superoxide anion radicals.
Huisman et al. [184] found that wine polyphenols and ethanol do not significantly scavenge superoxide nor affect endothelial nitric oxide production. Studies showed that flavonoids can induce oxidative damage and nick DNA via the production of radicals in the presence of Cu and O (2). Al, Zn, Ca, Mg and Cd have been found to stimulate phenoxyl radical-induced lipid peroxidation [185]. As a result of such enzymatic as well as non-enzymatic antioxidant reactions, phenoxyl radicals are formed as the primary oxidized products. Phenoxyl radicals can initiate lipid peroxidation. It is concluded that the prooxidant cytotoxicity of diet polyphenols is due to formation of ROS [186], role of phenoxyl radical/phenol redox couple [187], and stimulation by metals [185].
5. Bioavailability of dietary polyphenols
Polyphenols are the most abundant antioxidants in the human diet. They show a considerable structural diversity, which largely influences their bioavailability [188]. The biological properties of polyphenols depend on the amount consumed and on their bioavailability. Bioavailability appears to differ greatly between the various polyphenols, and the most abundant polyphenols in our diet are not necessarily those leading to the highest concentrations of active metabolites in target tissues [189]. Both isoflavones and phenolic acids like caffeic acid and gallic acid are the most well absorbed polyphenols, followed by catechins, flavanones, and quercetin glucosides, but with different kinetics. The least well-absorbed polyphenols are large molecular weight polyphenols such as the proanthocyanidins, the galloylated tea catechins, and the anthocyanins [190].
Ellagic acid was detected in human plasma at a maximum concentration (31.9 ng/mL) after 1 h postingestion [191]. Absorption of flavanols such as catechins was enhanced when tea polyphenols were administered as a green tea supplement in capsule form when consumed in the absence of food and led to a small but significant increase in plasma antioxidant activity compared with when tea polyphenols were consumed as black tea or green tea [192,193]. No differences were found in plasma EGCG concentrations and trolox equivalents determined by the trolox equivalent antioxidant capacity assay after administration as a single large dose in the form of either purified EGCG or as green tea extract (Polyphenon E) [194]. Hydroxytyrosol, the major olive oil phenolic compound, is dosedependently absorbed from olive oil [195]. Tuck et al. showed that hydroxytyrosol intravenously and orally administered oil-based dosings resulted in significantly greater elimination of the phenolics in urine within 24 h than the oral, aqueous dosing method. Oral bioavailability estimates of hydroxylInt. tyrosol when administered in an olive oil solution and when dosed as an aqueous solution was 99% and 75%, respectively [13].
Once absorbed, polyphenols are conjugated to glucuronide, sulphate and methyl groups in the gut mucosa and inner tissues. Non-conjugated polyphenols are virtually absent in plasma. Such reactions facilitate their excretion and limit their potential toxicity. EGCG and ECG were present in plasma mostly as the free form, whereas epicatechin and epigallocatechin were mostly present as the glucuronide and sulfate conjugates [192]. Recent data suggest that beta-glucosidases and maybe also lactase phlorizin hydrolase (LPH) in the small intestine are capable of hydrolysing flavonoid glucosides and these compounds are thus taken up as the free aglycon and not as the intact glycosides [196]. It has been reported that around 98% of hydroxytyrosol is present in plasma and urine in conjugated forms, mainly glucuronoconjugates, suggesting an extensive first pass intestinal/hepatic metabolism of the ingested primary forms [197–199] and the 3-O-glucuronide of hydroxytyrosol shows stronger activity as a radical scavenger than hydroxytyrosol itself [200]. The major metabolites identified in in vitro and in vivo studies were an Omethylated derivative of hydroxytyrosol, glucuronides of hydroxytyrosol and a novel glutathionyl conjugate of hydroxytyrosol [200,201]. It has been recently reported that hydroxytyrosol and its metabolites are capable of binding human LDL after olive oil ingestion [202].
The polyphenols reaching the colon are extensively metabolised by the microflora into a wide array of low molecular weight phenolic acids. It has been shown that the plasma concentrations of total metabolites ranged from 0 to 4 μmol/L with an intake of 50 mg aglycone equivalents, and the relative urinary excretion ranged from 0.3% to 43% of the ingested dose, depending on the polyphenol [189]. The biological properties of both conjugated derivatives and microbial metabolites will be essential to better assess the health effects of dietary polyphenols. Alternatively, some health effects of polyphenols may not require their absorption through the gut barrier. Their role as iron chelators in the gut lumen is briefly discussed. Tannic acid and catechin both interact with the gut but only catechin appears able to traverse the gut. In addition, they provide evidence for binding of tannic acid and catechin by endogenous proteins in the intestinal lumen. This may limit their absorption from the small intestine [203].
6. Conclusions
Consumption of polyphenol-rich fruits, vegetables, and beverages derived from plants, such as cocoa, red wine and tea, represents a diet beneficial to human health. Some dietary polyphenols possess antioxidative and anti-inflammatory properties, to some extent, contributing to their cancer chemopreventive potential. These phenolic substances have the ability to abrogate various biochemical processes induced or mediated by the tumor promoters. Some dietary polyphenols also induce apoptosis in premalignant or cancerous cells, and suppress growth and proliferation of various types of tumor cells via induction of apoptosis or arrest of a specific phase of the cell cycle.
However, the specific mechanism(s) by which these compounds affect human health remains unclear, despite extensive research conducted in this area in recent years. Most of that research has focused on the antioxidant properties of dietary polyphenols, which are well characterized and well established in vitro. The in vitro data often conflict with results obtained from in vivo studies on the antioxidant capacity of plasma or the resistance of plasma and lipoproteins to oxidation ex vivo after the consumption of polyphenols-rich foods by human subjects. These inconsistencies between the in vitro and the in vivo data are likely explained by the limited bioavailability of dietary polyphenols and their extensive metabolism in humans. Most of them exert multifacet action, and any clinical applications using these substances should be based on the precise understanding of the physiologically relevant action mechanisms.
Acknowledgements
This project was supported by a grant from the National Natural Science Foundation of P.R.China (No. 30472072)
References
- Gutteridge, J.M. Free radicals in diseases processes: a compilation of cause and consequence. Free Radic Res. Commun 1993, 19, 141–158. [Google Scholar]
- Kehrer, J.P. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol 1993, 23, 21–48. [Google Scholar]
- Becker, L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res 2004, 61, 461–470. [Google Scholar]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defences against oxidative stress. Free Radic. Res 1999, 31, 273–300. [Google Scholar]
- Masella, R.; Di Benedetto, R.; Vari, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem 2005, 16, 577–586. [Google Scholar]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R. N.; Beth Finn, M.; Holtzman, D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis 2006, 24, 506–515. [Google Scholar]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett 1997, 418, 152–156. [Google Scholar]
- Shen, S.Q; Zhang, Y.; Xiang, J.J.; Xiong, C.L. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J. Gastroenterol 2007, 13, 1953–1961. [Google Scholar]
- Molina, M.F.; Sanchez-Reus, I.; Iglesias, I.; Benedi, J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol. Pharm. Bull 2003, 26, 1398–1402. [Google Scholar]
- Chen, C.; Yu, R.; Owuor, E.D.; Kong, A.N. Activation of antioxidant response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res 2000, 23, 605–612. [Google Scholar]
- Butterfield, D.A.; Castegna, A.; Pocernich, C. B.; Drake, J.; Scapagninib, G.; Calabresec, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem 2002, 13, 444–461. [Google Scholar]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem 2007, 55, 5043–5049. [Google Scholar]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr 2001, 131, 1993–1996. [Google Scholar]
- Rice-Evans, C.A.; Mmiller, N.J.; Paganga, G. Antioxidant properties of phenolic compounas. Trends Plant Sci 1997, 2, 152–159. [Google Scholar]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem 2003, 51, 2813–2820. [Google Scholar]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr 2001, 40, 113–120. [Google Scholar]
- McAnlis, G.T.; McEneny, J.; Pearce, J.; Young, I.S. Absorption and antioxidant effects of quercetin from onions, in man. Eur. J. Clin. Nutr 1999, 53, 92–96. [Google Scholar]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr 2003, 57, 235–242. [Google Scholar]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med 2006, 41, 1727–1746. [Google Scholar]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr 2001, 131, 235–241. [Google Scholar]
- Henning, S.M.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.L.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem 2005, 16, 610–616. [Google Scholar]
- Widlansky, M.E.; Duffy, S.J.; Hamburg, N.M.; Gokce, N.; Warden, B.A.; Wiseman, S.; Keaney, J.F., Jr.; Frei, B.; Vita, J.A. Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Radic. Biol. Med 2005, 38, 499–506. [Google Scholar]
- Bell, J.R.; Donovan, J.L.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R. L.; Kasim-Karakas, S.E. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr 2000, 71, 103–108. [Google Scholar]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr 2002, 76, 798–804. [Google Scholar]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med 2006, 41, 1727–1746. [Google Scholar]
- Schwarz, D.; Roots, I. In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem. Biophys. Res. Commun 2003, 303, 902–907. [Google Scholar]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother 2006, 60, 536–540. [Google Scholar]
- Seeram, N. P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol 2007, 45, 328–336. [Google Scholar]
- Ray, P.S.; Maulik, G.; Cordis, G.A.; Bertelli, A.A.; Bertelli, A.; Das, D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med 1999, 27, 160–169. [Google Scholar]
- Zhang, Y.; Liu, Y.; Wang, T.; Li, B.; Li, H.; Wang, Z.; Yang, B. Resveratrol, a natural ingredient of grape skin:Antiarrhythmic efficacy and ionic mechanisms. Biochem. Biophys. Res. Commun 2006, 340, 1192–1199. [Google Scholar]
- Chung, KT.; Wong, TY.; Wei, CI.; Huang, YW.; Lin, Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr 1998, 38, 421–464. [Google Scholar]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar]
- Hong, J.; Smith, T.J.; Ho, C.T.; August, D.A.; Yang, C.S. Effects of purified green and black tea polyphenols on cyclooxygenase-and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem. Pharmacol 2001, 62, 1175–1183. [Google Scholar]
- Fki, I.; Sahnoun, Z.; Sayadi, S. Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J. Agric. Food Chem 2007, 55, 624–631. [Google Scholar]
- Kohyama, N.; Nagata, T.; Fujimoto, S.; Sekiya, K. Inhibition of arachidonate lipoxygenase activities by 2-(3, 4-dihydroxyphenyl) ethanol, a phenolic compound from olives. Biosci. Biotechnol. Biochem 1997, 61, 347–350. [Google Scholar]
- Du, Y.; Guo, H.; Lou, H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J. Agric. Food Chem 2007, 55, 1695–1701. [Google Scholar]
- Appiah-Opong, R.; Commandeur, J.N.; van Vugt-Lussenburg, B.; Vermeulen, N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol 2000, 130, 1115–1123. [Google Scholar]
- Nishinaka, T.; Ichijo, Y.; Ito, M.; Kimura, M.; Katsuyama, M.; Iwata, K.; Miura, T.; Terada, T.; Yabe-Nishimura, C. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol Lett 2007, 170, 238–247. [Google Scholar]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; Ferrandiz, M.L.; Bustos, G.; Paya, M.; Gunasegaran, R.; Alcaraz, M.J. Effects of flavonoids on Naja naja and human recombinant synovial phospholipase A2 and inflammatory responses in mice. Life Sci 1994, 54, 333–338. [Google Scholar]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J. Nutr. Biochem 2004, 15, 722–729. [Google Scholar]
- Kerry, N.; Rice-Evans, C. Inhibition of peroxynitrite-mediated oxidation of dopamine by flavonoid and phenolic antioxidants and their structural relationship. J. Neurochem 1999, 73, 247–253. [Google Scholar]
- Alía, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol 2006, 212, 110–118. [Google Scholar]
- Valerio, L.G., Jr; Kepa, J.K.; Pickwell, G.V.; Quattrochi, L.C. Induction of human NAD(P)H:quinone oxidoreductase (NQO1) gene expression by the flavonol quercetin. Toxicol. Lett 2001, 119, 49–57. [Google Scholar]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med 2004, 10, 549–557. [Google Scholar]
- Scharf, G; Prustomersky, S.; Knasmuller, S.; Schulte-Hermann, R.; Huber, W.W. Enhancement of glutathione and g-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemoprotective plant-derived food and beverage components in the human hepatoma cell line HepG2. Nutr. Cancer 2003, 45, 74–83. [Google Scholar]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med 2007, 42, 1690–1703. [Google Scholar]
- Cadenas, S.; Barja, G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic. Biol. Med 1999, 26, 1531–1537. [Google Scholar]
- Jang, M.; Pezzuto, J.M. Effects of resveratrol on 12-O-tetradecanoylphorbol -13-acetate -induced oxidative events and gene expression in mouse skin. Cancer Lett 1998, 134, 81–89. [Google Scholar]
- Dubuisson, J.G.; Dyess, D.L.; Gaubatz, J.W. Resveratrol modulates human mammary epithelial cell O-acetyltransferase, sulfotransferase, and kinase activation of the heterocyclic amine carcinogen N-hydroxy-PhIP. Cancer Lett 2002, 182, 27–32. [Google Scholar]
- Ciolino, H.P.; Yeh, G.C. Inhibition of aryl hydrocarbon induced cytochrome P-4501A1 enzyme activity and CYP1A1 expression by resveratrol. Mol. Pharmacol 1999, 56, 760–767. [Google Scholar]
- Casper, R.F.; Quesne, M.; Rogers, I.M.; Shirota, T.; Jolivet, A.; Milgrom, E.; Savouret, J.F. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol. Pharmacol 1999, 56, 784–790. [Google Scholar]
- Schewe, T.; Sadik, C.; Klotz, L.O.; Yoshimoto, T.; Kuhn, H.; Sies, H. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol. Chem 2001, 382, 1687–1696. [Google Scholar]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem 1998, 273, 21875–21882. [Google Scholar]
- Li, Y.T.; Shen, F.; Liu, B.H.; Cheng, G.F. Resveratrol inhibits matrix metalloproteinase-9 transcription in U937 cells. Acta Pharmacol. Sin 2003, 24, 1167–1171. [Google Scholar]
- Kaga, S.; Zhan, L.; Matsumoto, M.; Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell. Cardiol 2005, 39, 813–822. [Google Scholar]
- Cullen, J.P.; Morrow, D.; Jin, Y.; von Offenberg Sweeney, N.; Sitzmann, J.V.; Cahill, P.A.; Redmond, E.M. Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis 2007, in press. [Google Scholar]
- Wang, S.; Wang, X.; Yan, J.; Xie, X.; Fan, F.; Zhou, X.; Han, L.; Chen, J. Resveratrol inhibits proliferation of cultured rat cardiac fibroblasts: Correlated with NO-cGMP signaling pathway. Eur. J. Pharmacol 2007, 567, 26–35. [Google Scholar]
- Steffen, Y.; Wiswedel, I.; Peter, D.; Schewe, T.; Sies, H. Cytotoxicity of myeloperoxidase/nitrite-oxidized low-density lipoprotein toward endothelial cells is due to a high 7β-hydroxycholesterol to 7-ketocholesterol ratio. Free Radic. Biol. Med 2006, 41, 1139–1150. [Google Scholar]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res 1995, 78, 151–160. [Google Scholar]
- Léger, C.L.; Carbonneau, M.A.; Michel, F.; Mas, E.; Monnier, L.; Cristol, J.P.; Descomps, B. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur. J. Clin. Nutr 2005, 59, 727–730. [Google Scholar]
- de La Puerta, R.; Ruiz-Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol 1999, 57, 445–449. [Google Scholar]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol 2003, 23, 622–629. [Google Scholar]
- Manna, C.; Migliardi, V.; Golino, P.; Scognamiglio, A.; Galletti, P.; Chiariello, M.; Zappia, V. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J. Nutr. Biochem 2004, 15, 461–466. [Google Scholar]
- Gouedard, C.; Barouki, R.; Morel, Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell Biol 2004, 24, 5209–5222. [Google Scholar]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Kumar, N.; Nair, R.E.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFN gamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar]
- Myhrstad, M.C.; Carlsen, H.; Nordstrom, O.; Blomhoff, R.; Moskaug, J.O. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med 2002, 32, 386–393. [Google Scholar]
- Lo, H.M.; Hung, C.F.; Huang, Y.Y.; Wu, W.B. Tea polyphenols inhibit rat vascular smooth muscle cell adhesion and migration on collagen and laminin via interference with cell-ECM interaction. J. Biomed. Sci 2007, in press. [Google Scholar]
- Mizushige, T.; Mizushige, K.; Miyatake, A.; Kishida, T.; Ebihara, K. Inhibitory effects of soy isoflavones on cardiovascular collagen accumulation in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 48–52. [Google Scholar]
- Tikkanen, M.J.; Adlercreutz, H. Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease prevention? Biochem.Pharmacol 2000, 60, 1–5. [Google Scholar]
- Schramm, D.D.; Wang, J.F.; Holt, R.R.; Ensunsa, J.L.; Gonsalves, J.L.; Lazarus, S.A.; Schmitz, H.H.; German, J.B.; Keen, C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr 2001, 73, 36–40. [Google Scholar]
- Dedoussis, G.V.; Kaliora, A.C.; Psarras, S.; Chiou, A.; Mylona, A.; Papadopoulos, N.G.; Andrikopoulos, N.K. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis 2004, 174, 293–303. [Google Scholar]
- Sato, M.; Bagchi, D.; Tosaki, A.; Das, D.K. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia–reperfusion-induced activation of JNK-1 and c-JUN. Free Radic. Biol. Med 2001, 31, 729–737. [Google Scholar]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 7217–7222. [Google Scholar]
- Chavez, E.; Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl (4) treatment in rats. J. Appl. Toxicol 2007, in press. [Google Scholar]
- Bastianetto, S.; Brouillette, J.; Quirion, R. Neuroprotective effects of natural products: interaction with intracellular kinases, amyloid peptides and a possible role for transthyretin. Neurochem. Res 2007, in press. [Google Scholar]
- Okawara, M.; Katsuki, H.; Kurimoto, E.; Shibata, H.; Kume, T.; Akaike, A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol 2007, 73, 550–560. [Google Scholar] [Green Version]
- Kim, S.J.; Jeong, H.J.; Lee, K.M.; Myung, N.Y.; An, N.H.; Mo Yang, W; Kyu Park, S.; Lee, H.J.; Hong, S.H.; Kim, H.M.; Um, J.Y. Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem 2007, in press. [Google Scholar]
- de Boer, V.C.; de Goffau, M.C.; Arts, I.C.; Hollman, P.C.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev 2006, 127, 618–627. [Google Scholar]
- Levites, Y.; Amit, T.; Youdim, M.B.; Mandel, S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin-3-gallate neuron-protective action. J. Biol. Chem 2002, 277, 30574–30580. [Google Scholar]
- Mercer, L. D.; Kelly, B.L.; Horne, M. K.; Beart, P.M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem. Pharmacol 2005, 69, 339–345. [Google Scholar]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), cjun and caspase-3. Biochem. J 2001, 358, 547–557. [Google Scholar]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem 2007, 102, 1095–1104. [Google Scholar]
- Mao, T.K.; Powell, J.; Van de Water, J.; Keen, C.L.; Schmitz, H.H.; Hammerstone, J.F.; Eric Gershwin, M. The effect of cocoa procyanidins on the transcription and secretion of interleukin lβ in peripheral blood mononuclear cells. Life Sci 2000, 66, 1377–1386. [Google Scholar]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Sasaki, S.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate induces apoptosis of monocytes. J. Allergy Clin. Immunol 2005, 115, 186–191. [Google Scholar]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J. Allergy Clin. Immunol 2004, 113, 1211–1217. [Google Scholar]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci 2007, 1095, 554–563. [Google Scholar]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NF-κB in macrophages by resveratrol. Br. J. Pharmacol 1999, 126, 673–680. [Google Scholar]
- Nonn, L.; Duong, D.; Peehl, D.M. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis 2007, 28, 1188–1196. [Google Scholar]
- Gerritsen, M.E.; Carley, W.W.; Ranges, G.E.; Shen, C.P.; Phan, S.A.; Ligon, G.F.; Perry, C.A. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am. J. Pathol 1995, 147, 278–292. [Google Scholar]
- Choi, J.S.; Choi, Y.J.; Park, S.H.; Kang, J.S.; Kang, Y.H. Flavones mitigate tumor necrosis factor-alpha-induced adhesion molecule upregulation in cultured human endothelial cells: role of nuclear factor-kappa B. J. Nutr 2004, 134, 1013–1019. [Google Scholar]
- van Meeteren, M.E.; Hendriks, J.J.; Dijkstra, C.D.; van Tol, E.A. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem. Pharmacol 2004, 67, 967–975. [Google Scholar]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem 2002, 13, 282–288. [Google Scholar]
- Camacho-Barquero, L.; Villegas, I.; Sanchez-Calvo, J.M.; Talero, E.; Sanchez-Fidalgo, S.; Motilva, V.; Alarcon de la Lastra, C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol 2007, 7, 333–342. [Google Scholar]
- Rahman, I.; Biswas, SK.; Kirkham, PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol 2006, 72, 1439–1452. [Google Scholar]
- Kim, H.Y.; Park, E.J.; Joe, E.H.; Jou, I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol 2003, 171, 6072–6079. [Google Scholar]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev 2002, 11, 351–358. [Google Scholar]
- Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Cottarelli, A.; Falchetti, R.; Ravagnan, G.; Bonmassar, E. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J. Exp. Clin. Cancer Res 2006, 25, 189–193. [Google Scholar]
- Kuo, P.L.; Chiang, L.C.; Lin, C.C. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in HepG2 cells. Life Sci 2002, 72, 23–34. [Google Scholar]
- Pozo-Guisado, E.; Lorenzo-Benayas, M.J.; Fernandez-Salguero, P.M. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int. J. Cancer 2004, 109, 167–173. [Google Scholar]
- Kundu, J.K.; Chun, K.S.; Kim, S.O.; Surh, Y.J. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. Biofactors 2004, 21, 33–39. [Google Scholar]
- Yoon, S.H.; Kim, Y.S.; Ghim, S.Y.; Song, B.H.; Bae, Y.S. Inhibition of protein kinase CKII activity by resveratrol, a natural compound in red wine and grapes. Life Sci 2002, 71, 2145–2152. [Google Scholar]
- Slater, S.J.; Seiz, J.L.; Cook, A.C.; Stagliano, B.A.; Buzas, C.J. Inhibition of protein kinase C by resveratrol. Biochim. Biophys. Acta 2003, 1637, 59–69. [Google Scholar]
- Li, H.; Cheng, Y.; Wang, H.; Sun, H.; Liu, Y.; Liu, K.; Peng, S. Inhibition of nitrobenzene-induced DNA and hemoglobin adductions by dietary constituents. Appl. Radiat. Isot 2003, 58, 291–298. [Google Scholar]
- Grace, S.C.; Salgo, M.G.; Pryor, W.A. Scavenging of peroxynitrite by a phenolic/peroxidase system prevents oxidative damage to DNA. FEBS Lett 1998, 426, 24–28. [Google Scholar]
- Lee, L.T.; Huang, Y.T.; Hwang, J.J.; Lee, P.P.; Ke, F.C.; Nair, M.P.; Kanadaswam, C.; Lee, M.T. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res 2002, 22, 1615–1627. [Google Scholar]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun 2004, 317, 269–275. [Google Scholar]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 2007, 67, 3853–3861. [Google Scholar]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xL. Carcinogenesis 2002, 23, 143–150. [Google Scholar]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.; Kondo, Y.; Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and ERK signaling pathways. Mol. Pharmacol 2007, 72, 29–39. [Google Scholar]
- Nardini, M.; Leonardi, F.; Scaccini, C.; Virgili, F. Modulation of ceramide-induced NF-κB binding activity and apoptotic response by caffeic acid in U937 cells: comparison with other antioxidants. Free Radic. Biol. Med 2001, 30, 722–733. [Google Scholar]
- Naasani, I.; Oh-Hashi, F.; Oh-Hara, T.; Feng, W.Y.; Johnston, J.; Chan, K.; Tsuruo, T. Blocking telomerase by dietary polyphenols is a major mechanisms for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res 2003, 63, 824–830. [Google Scholar]
- Jung, J.Y.; Mo, H.C.; Yang, K.H.; Jeong, Y.J.; Yoo, H.G.; Choi, N.K.; Oh, W.M.; Oh, H.K.; Kim, S.H.; Lee, J.H.; Kim, H.J.; Kim, W.J. Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci 2007, 80, 1355–1363. [Google Scholar]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. Biochim. Biophys. Acta 2006, 1760, 283–289. [Google Scholar]
- Choi, Y.J.; Jeong, Y.J.; Lee, Y.J.; Kwon, H.M.; Kang, Y.H. (−) Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J. Nutr 2005, 135, 707–713. [Google Scholar]
- Bharti, A.C.; Donato, N.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol 2003, 171, 3863–3871. [Google Scholar]
- Yuste, P.; Longstaff, M.; McCorquodale, C. The effect of proanthocyanidin-rich hulls and proanthocyanidin extracts from bean (Vicia faba L.) hulls on nutrient digestibility and digestive enzyme activities in young chicks. Br. J. Nutr 1992, 67, 57–65. [Google Scholar]
- Longstaff, M.; McNab, J.M. The inhibitory effects of hull polysaccharides and tannins of field beans (Vicia faba L.) on the digestion of amino acids, starch and lipid and on digestive enzyme activities in young chicks. Br. J. Nutr 1991, 65, 199–216. [Google Scholar]
- Sbarra, V.; Ristorcelli, E.; Petit-Thevenin, J.L.; Teissedre, P.L.; Lombardo, D.; Verine, A. In vitro polyphenol effects on activity, expression and secretion of pancreatic bile salt-dependent lipase. Biochim. Biophys. Acta 2005, 1736, 67–76. [Google Scholar]
- Aggarwal, B.B.; Shisodia, S. Suppression of the nuclear factorkappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann. N.Y. Acad. Sci 2004, 1030, 434– 441. [Google Scholar]
- Hanamura, T.; Hagiwara, T.; Kawagishi, H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC.) fruit. Biosci. Biotechnol. Biochem 2005, 69, 280–286. [Google Scholar]
- Youn, H.S.; Saitoh, S.I.; Miyake, K.; Hwang, D.H. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem. Pharmacol 2006, 72, 62–69. [Google Scholar]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharmacol 2000, 60, 1665–1676. [Google Scholar]
- Kang, G.; Kong, P.J.; Yuh, Y.J.; Lim, S.Y.; Yim, S.V.; Chun, W.; Kim, S.S. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappaB bindings in BV2 microglial cells. J. Pharmacol. Sci 2004, 94, 325–328. [Google Scholar]
- Kluth, D.; Banning, A.; Paur, I.; Blomhoff, R.; Brigelius-Flohe, R. Modulation of pregnane X receptor-and electrophile responsive element-mediated gene expression by dietary polyphenolic compounds. Free Radic. Biol. Med 2007, 42, 315–325. [Google Scholar]
- Meeran, S.M.; Katiyar, S.K. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp. Dermatol 2007, 16, 405–415. [Google Scholar]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol. Cancer Ther 2007, 6, 995–1005. [Google Scholar]
- Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Modulation of pro-survival Akt/PKB and ERK1/2 signalling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem 2003, 278, 34783–34793. [Google Scholar]
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem 2006, 281, 27335–27345. [Google Scholar]
- Chen, C.Y.; Jang, J.H.; Li, M.H.; Surh, Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun 2005, 331, 993–1000. [Google Scholar]
- Oak, M.H.; Chataigneau, M.; Keravis, T.; Chataigneau, T.; Beretz, A.; Andriantsitohaina, R.; Stoclet, J.C.; Chang, S.J.; Schini-Kerth, V.B. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arterioscler. Thromb. Vasc. Biol 2003, 23, 1001–1007. [Google Scholar]
- Oak, M.H.; El Bedoui, J.; Anglard, P.; Schini-Kerth, V.B. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation 2004, 110, 1861–1867. [Google Scholar]
- Iijima, K.; Yoshizumi, M.; Hashimoto, M.; Akishita, M.; Kozaki, K.; Ako, J.; Watanabe, T.; Ohike, Y.; Son, B.; Yu, J.; Nakahara, K.; Ouchi, Y. Red wine polyphenols inhibit vascular smooth muscle cell migration through two distinct signaling pathways. Circulation 2002, 105, 2404–2410. [Google Scholar]
- Ndiaye, M.; Chataigneau, T.; Chataigneau, M.; Schini-Kerth, V.B. Red wine polyphenols induce EDHF-mediated relaxations in porcine coronary arteries through the redox-sensitive activation of the PI3-kinase/Akt pathway. Br. J. Pharmacol 2004, 142, 1131–1136. [Google Scholar]
- Martin, S.; Andriambeloson, E.; Takeda, K.; Andriantsitohaina, R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol 2002, 135, 1579–1587. [Google Scholar]
- Jiménez, R.; López-Sepúlveda, R.; Kadmiri, M.; Romero, M.; Vera, R.; Sánchez, M.; Vargas, F.; O’valle, F.; Zarzuelo, A.; Dueñas, M.; Santos-Buelga, C.; Duarte, J. Polyphenols restore endothelial function in DOCA-salt hypertension: Role of endothelin-1 and NADPH oxidase. Free Radic. Biol. Med 2007, 43, 462–473. [Google Scholar]
- Khan, N.Q.; Lees, D.M.; Douthwaite, J.A.; Carrier, M.J.; Corder, R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin. Sci. (Lond) 2002, 103 Suppl 48, 72S–75S. [Google Scholar]
- Wollny, T.; Chabielska, E.; Malinowska-Zaprzałka, M.; Nazarko, J.; Rozmysłowicz-Szermińska, W.; Buczko, W. Effects of Bulgarian red and white wines on primary hemostasis and experimental thrombosis in rats. Pol. J. Pharmacol 2003, 55, 1089–1096. [Google Scholar]
- Dell’Agli, M.; Busciala, A.; Bosisio, E. Vascular effects of wine polyphenols. Cardiovasc. Res 2004, 63, 593–602. [Google Scholar]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett 2004, 574, 176–180. [Google Scholar]
- Kim, J.A.; Formoso, G.; Li, Y.; Potenza, M.A.; Marasciulo, F.L.; Montagnani, M.; Quon, M.J. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J. Biol. Chem 2007, 282, 13736–13745. [Google Scholar]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Düsterhöft, T.; Baumann, G.; Stangl, K.; Stangl, V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem 2004, 279, 6190–6195. [Google Scholar]
- Maiti, T.K.; Chatterjee, J.; Dasgupta, S. Effect of green tea polyphenols on angiogenesis induced by an angiogenin-like protein. Biochem. Biophys. Res. Commun 2003, 308, 64–67. [Google Scholar]
- Kalin, R.; Righi, A.; Del Rosso, A.; Bagchi, D.; Generini, S.; Cerinic, M.M.; Das, D.K. Activin, a grape seed-derived proanthocyanidin extract, reduces plasma levels of oxidative stress and adhesion molecules (ICAM-1, VCAM-1 and E-selectin) in systemic sclerosis. Free Radic. Res 2002, 36, 819–825. [Google Scholar]
- Sen, C.K.; Bagchi, D. Regulation of inducible adhesion molecule expression in human endothelial cells by grape seed proanthocyanidin extract. Mol. Cell Biochem 2001, 216, 1–7. [Google Scholar]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett 2003, 555, 597–600. [Google Scholar]
- Alvarez, P.; Alvarado, C.; Puerto, M.; Schlumberger, A.; Jiménez, L.; De la Fuente, M. Improvement of leukocyte functions in prematurely aging mice after five weeks of diet supplementation with polyphenol-rich cereals. Nutrition 2006, 22, 913–921. [Google Scholar]
- Bhattacharyya, S.; Mandal, D.; Saha, B.; Sen, G.S.; Das, T.; Sa, G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J.Biol.Chem 2007, 282, 15954–15964. [Google Scholar]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R.; Sawada, J.; Goda, Y.; Maitani, T. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett 2005, 579, 4485–4491. [Google Scholar]
- Kanda, T.; Akiyama, H.; Yanagida, A.; Tanabe, M.; Goda, Y.; Toyoda, M.; Teshima, R.; Saito, Y. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci. Biotechnol. Biochem 1998, 62, 1284–1289. [Google Scholar]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (London) 2007, 4, 8. [Google Scholar]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett 2005, 579, 1653–1657. [Google Scholar]
- Kobayashi, Y.; Suzuki, M.; Hideo, S.; Arai, S.; Yukihiko, H.; Suzuki, K.; Miyamoto, Y.; Shimizu, M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J. Agric. Food Chem 2000, 48, 5618–5623. [Google Scholar]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of SVCT1 and GLUT2, intestinal transporters for vitamin C and glucose. J. Biol. Chem 2002, 277, 15252–15260. [Google Scholar]
- Yoshikawa, M.; Nishida, N.; Shimoda, H.; Takada, M.; Kawahara, Y.; Matsuda, H. Polyphenol constituents from Salacia species: quantitative analysis of mangiferin with alpha-glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi 2001, 121, 371–378. [Google Scholar]
- McDougall, GJ.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibits alpha-amylase and alpha-glucosidase. J. Agric. Food Chem 2005, 53, 2760–2766. [Google Scholar]
- Bhat, K.P.; Lantvit, D.; Christov, K.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res 2001, 61, 7456–7463. [Google Scholar]
- Bhat, K.P.; Pezzuto, J.M. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 2001, 61, 6137–6144. [Google Scholar]
- Otake, Y.; Nolan, A.L.; Walle, U.K.; Walle, T. Quercetin and resveratrol potently reduce estrogen sulfotransferase activity in normal human mammary epithelial cells. J. Steroid Biochem. Mol. Biol 2000, 73, 265–270. [Google Scholar]
- Reidenberg, M.M. Environmental inhibition of 11b-hydroxysteroid dehydrogenase. Toxicology 2000, 144, 107–111. [Google Scholar]
- Kuo, P.L.; Chiang, L.C.; Lin, C.C. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in HepG2 cells. Life Sci 2002, 72, 23–34. [Google Scholar]
- Ahmad, N.; Adhami, V. M.; Afaq, F.; Feyes, D. K.; Mukhtar, H. Resveratrol causesWAF-1/p21-mediated G (1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res 2001, 7, 1466–1473. [Google Scholar]
- Wolter, F.; Akoglu, B.; Clausnitzer, A.; Stein, J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J. Nutr 2001, 131, 2197–2203. [Google Scholar]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem. Biophys. Res. Commun 2001, 288, 579–585. [Google Scholar]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M. E.; Xiao, D.; Weinstein, I. B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res 2002, 8, 893–903. [Google Scholar]
- Poussier, B.; Cordova, A.C.; Becquemin, J.P.; Sumpio, B.E. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J. Vasc. Surg 2005, 42, 1190–1197. [Google Scholar]
- Larrosa, M; Tomas-Barberan, F.A.; Espin, J.C. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E, and B1 in human SK-Mel-28 melanoma cells. J. Agric. Food Chem 2003, 51, 4576–4584. [Google Scholar]
- Meeran, S.M.; Katiyar, S.K. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp. Dermatol 2007, 16, 405–415. [Google Scholar]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol 2004, 287, L774–L783. [Google Scholar]
- Rahman, I.; Kilty, I. Antioxidant therapeutic targets in COPD. Curr. Drug Targets 2006, 7, 707–720. [Google Scholar]
- Xu, M.; Deng, B.; Chow, Y.L.; Zhao, Z.Z.; Hu, B. Effects of curcumin in treatment of experimental pulmonary fibrosis: a comparison with hydrocortisone. J Ethnopharmacol 2007, 112, 292–299. [Google Scholar]
- Sakagami, Y.; Sawabe, A.; Komemushi, S.; All, Z.; Tanaka, T.; Iliya, I.; Iinuma, M. Antibacterial activity of stilbene oligomers against vancomycin-resistant Enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) and their synergism with antibiotics. Biocontrol Sci 2007, 12, 7–14. [Google Scholar]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother 2004, 48, 4892–4894. [Google Scholar]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Nair, H.N.; Chawda, R.; Shanahan, T.; Schwartz, S.A. Grape seed extract proanthocyanidins downregulate HIV-1 entry coreceptors, CCR2b, CCR3 and CCR5 gene expression by normal peripheral blood mononuclear cells. Biol. Res 2002, 35, 421–431. [Google Scholar]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic. Biol. Med 2001, 31, 38–42. [Google Scholar]
- Ray, S.D.; Kumar, M.A.; Bagchi, D. A novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch. Biochem. Biophys 1999, 369, 42–58. [Google Scholar]
- Wong, M.C.; Portmann, B.; Sherwood, R.; Niemela, O.; Koivisto, H.; Parkkila, S.; Trick, K.; L’abbe, M.R.; Wilson, J.; Dash, P.R.; Srirajaskanthan, R.; Preedy, V.R.; Wiseman, H. The cytoprotective effect of alpha-tocopherol and daidzein against d-galactosamine-induced oxidative damage in the rat liver. Metabolism 2007, 56, 865–875. [Google Scholar]
- Kuzu, N.; Metin, K.; Dagli, A.F.; Akdemir, F.; Orhan, C.; Yalniz, M.; Ozercan, I.H.; Sahin, K.; Bahcecioglu, I.H. Protective role of genistein in acute liver damage induced by carbon tetrachloride. Mediators Inflamm 2007, 2007, 36381. [Google Scholar]
- Rucinska, A.; Kirko, S.; Gabryelak, T. Effect of the phytoestrogen, genistein-8-C-glucoside, on Chinese hamster ovary cells in vitro. Cell Biol. Int 2007, in press. [Google Scholar]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar]
- Rietjens, I.M.C.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J. H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol 2002, 11, 321–333. [Google Scholar]
- Huisman, A.; van de Wiel, A.; Rabelink, T.J.; van Faassen, E.E. Wine polyphenols and ethanol do not significantly scavenge superoxide nor affect endothelial nitric oxide production. J. Nutr. Biochem 2004, 15, 426–432. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res 2004, 24, 563–569. [Google Scholar]
- Nemeikaite-Ceniene, A.; Imbrasaite, A.; Sergediene, E.; Cenas, N. Quantitative structure-activity relationships in prooxidant cytotoxicity of polyphenols: role of potential of phenoxyl radical/phenol redox couple. Arch. Biochem. Biophys 2005, 441, 182–190. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr 2004, 79, 727–747. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr 2005, 81, 230S–242S. [Google Scholar]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med 2003, 35, 837–844. [Google Scholar]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res 2005, 11, 4627–4633. [Google Scholar]
- Henning, S.M.; Niu, Y.; Lee, N.H.; Thames, G.D.; Minutti, R.R.; Wang, H.; Go, V.L.; Heber, D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr 2004, 80, 1558–1564. [Google Scholar]
- Henning, S.M.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.L.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem 2005, 16, 610–616. [Google Scholar]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett 2000, 468, 159–160. [Google Scholar]
- Rasmussen, S.E.; Breinholt, V.M. Non-nutritive bioactive food constituents of plants: bioavailability of flavonoids. Int. J. Vitam. Nutr. Res 2003, 73, 101–111. [Google Scholar]
- Covas, M.I. Olive oil and the cardiovascular system. Pharmacol. Res 2007, 55, 175–186. [Google Scholar]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem 2003, 49, 945–952. [Google Scholar]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar]
- Tuck, K.L.; Hayball, P.J.; Stupans, I. Structural characterization of the metabolites of hydroxytyrosol, the principal phenolic component in olive oil in rats. J. Agric. Food Chem 2002, 50, 2404–2409. [Google Scholar]
- Corona, G.; Tzounis, X.; Assunta Dessi, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P. The fate of olive oil polyphenols in the gastrointestinal tract: implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res 2006, 40, 647–658. [Google Scholar]
- de la Torre-Carbot, K.; Jauregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Covas, M.I.; Casals, I.; López-Sabater, M.C. Rapid high-performance liquid chromatography-electrospray ionization tandem mass spectrometry method for qualitative and quantitative analysis of virgin olive oil metabolites in human low-density lipoproteins. J. Chromatogr. A 2006, 1116, 69–75. [Google Scholar]
- Carbonaro, M.; Grant, G.; Pusztai, A. Evaluation of polyphenol bioavailability in rat small intestine. Eur. J. Nutr 2001, 40, 84–90. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.