Abstract
Alcohol-associated hepatitis (AH) is the most severe clinical manifestation of alcohol-associated liver disease. Corticosteroids are the only disease-specific therapy shown to improve short-term survival. Currently, no non-invasive markers are available to predict patient response to corticosteroids or long-term survival in AH. This study investigates whether surface antigens on plasma extracellular vesicles (EVs), key mediators of intercellular communication, can reflect the underlying immune dysregulation in AH and serve as prognostic markers. Patients with AH were prospectively enrolled between 2020 and 2024. Blood samples were collected before corticosteroid initiation during the first 24 h of hospitalization. EVs were characterized using nanoparticle tracking analysis, cryo-electron microscopy, and flow cytometry. Interleukin-6 (IL-6), soluble (s)CD62p, Circulating Vascular Cell Adhesion Molecule-1 (sVCAM), tumor necrosis factor receptor superfamily member 1 (TNRFS1a), and Intercellular Adhesion Molecule 1 (ICAM-1) were quantified by ELISA. Key outcome variables included response to corticosteroids and mortality. A total of 46 patients with AH and 28 healthy donors (HD) were included. EV concentration was significantly higher in AH patients than in HD (9.3 × 1011 [IQR 4–24] versus 2.4 × 1011 [IQR 2–4], p = 0.03). Specific EV antigens were associated with key clinical outcomes: CD20 and CD2 levels differed between patients with or without infections (bacterial, viral, and fungal) developed during hospitalization; CD40 and CD146 were elevated in patients who developed acute kidney injury. EVs enriched in monocyte (CD14) and T-reg (CD25) markers were associated with plasma IL-6 levels, while endothelial markers CD105 and CD146 correlated with sVCAM and sCD62p. EVs enriched in platelet (CD49e) and endothelial (CD31) markers were associated with corticosteroid response, whereas EVs enriched with endothelial (CD105 and CD146) and B lymphocyte (CD19) markers were associated with mortality. Overall, EVs enriched in endothelial and monocyte markers may represent a candidate non-invasive tool for predicting corticosteroid response and mortality in AH, aiding risk stratification and early identification of non-responders for timely transplant evaluation.
1. Introduction
Alcohol-associated liver disease (ALD) is the leading cause of advanced liver disease worldwide [1,2]. Alcohol-associated hepatitis (AH) is the most severe and complex manifestation of ALD. It is associated with systemic inflammatory response syndrome (SIRS) [3], immune dysfunction (i.e., the dysregulation of many cells, subpopulations, and the suppression of both innate and adaptive immune responses in liver cells) [4,5], favoring bacterial infections [6], and acute kidney injury (AKI) [7]. A third of AH patients develop acute-on-chronic liver failure [8], a condition with high short-term mortality (20 to 50% at three months) [9]. Abstinence remains the cornerstone of treatment, although alcohol relapse is frequent after an episode of AH [10]. Corticosteroids are currently the only disease-specific therapy shown to improve short-term survival, within 28 days, in selected patients [11,12,13]. However, their use further amplifies the underlying immune dysfunction and is associated with an increased risk of severe infections, which significantly impacts prognosis and limits their clinical benefit [14,15]. Therefore, careful patient selection is critical to balance potential survival benefits against the heightened risk of infectious complications [16,17,18]. Currently, no non-invasive biomarkers are available to reliably predict patient response to corticosteroids or long-term survival in AH. Moreover, several novel therapeutic strategies have been explored, including modulation of inflammatory pathways, targeting gut–liver axis dysfunction, antioxidant therapies, and regenerative or cell-based approaches; however, none have demonstrated consistent clinical benefit to date [19].
AH is marked by complex immune activation, including neutrophil, monocyte/macrophage, and T-cell recruitment, cytokine storm, and paradoxical immunosuppression [5]. Concurrently, elevated levels of endothelial dysfunction markers (Vascular Cell Adhesion Molecule 1 (VCAM-1), Intercellular Adhesion Molecule 1 (ICAM-1), Cluster of Differentiation 146 (also known as MCAM-CD146), and Vascular Endothelial Growth Factor A (VEGF-A) correlate with disease severity, portal hypertension, and mortality [20]. Notably, these markers remain persistently elevated for up to 12 months, even after alcohol abstinence, indicating sustained endothelial injury [21]. However, chronic endothelial activation has not yet been recognized as a contributing mechanism to the persistent immune dysfunction observed after an episode of AH. Advancing effective therapies in AH will require a deeper understanding of the cellular crosstalk between immune cells and endothelial dysfunction that underpins AH and its clinical complications.
Promising, yet less investigated markers in ALD are extracellular vesicles (EVs) [22,23]. EVs are nano-sized membrane-bound particles, encompassing small and large vesicle populations, released by cells under physiological or pathological conditions and carrying cargo that reflects their cellular origin and functional state [24,25]. EVs, which are found in biological fluids, may serve as circulating biomarkers reflecting immune and endothelial alterations rather than as direct mediators of disease pathogenesis, while also participating in cell-to-cell communication [24,26]. The release of EVs increased, and their composition differs in ALD and AH patients compared to healthy individuals [16,27,28]. EVs may modify transcription in neighboring hepatocytes and non-parenchymal cells, modulating liver inflammation and fibrosis [27,28,29,30]. EVs in AH are enriched with specific microRNAs, especially miR192, miR122, and miR30a, with miR192 showing the strongest diagnostic potential [29]. Further studies have documented that EVs also carry damage-associated molecular patterns (DAMPs), activate macrophages via TLR9, and upregulate pro-inflammatory cytokine production (e.g., IL-1β and TNFα), suggesting that EVs may consequently play a crucial role in cellular crosstalk during an AH episode [28,29,31].
However, it remains unknown whether the surface antigen of circulating EVs, particularly the enrichment of EVs with immune and endothelial markers, can serve as non-invasive indicators of the interplay between immune dysregulation and endothelial dysfunction in AH. Specifically, the potential association between EV enrichment in selected surface antigens and clinically relevant outcomes, such as response to corticosteroid therapy, and short- and long-term prognosis, has not yet been established.
Therefore, the aim of this study was to characterize the enrichment of immune- and endothelial-associated surface antigens on plasma EVs in patients with AH, and to evaluate their association with immune–endothelial crosstalk, corticosteroid response, short- and long-term clinical outcomes.
2. Results
During the study period, 58 patients with a clinical suspicion of AH were admitted to Hospital de la Santa Creu i Sant Pau. Among them, 46 patients were included in the study (Supplementary Figure S1). The epidemiological, clinical, laboratory, and hemodynamic characteristics of the included patients are shown in Table 1. Patients with AH were predominantly young males, with 76% reporting tobacco use and 35% presenting features of metabolic syndrome. For 76% of patients, this was their first admission, with ascites as the initial decompensation in 41%. Of the patients included, 76% had biopsy-confirmed AH. The Model for End-Stage Liver Disease (MELD), the Maddrey DF score, and ABIC scores were similar regardless of histological confirmation. Hemodynamic assessments were performed on 87% of the cohort.

Table 1.
Baseline demographic, clinical, and hemodynamic characteristics of patients with alcohol-associated hepatitis in the study cohort.
2.1. Patients with AH Had Increased Numbers of Circulating EVs with a Different EV Antigen Profile Compared to Healthy Donors
A quantitative characterization by Nanoparticle Tracking Analysis (NTA) showed a significantly increased size heterogeneity (Supplementary Figure S2A,B) and total number (Supplementary Figure S2C) of circulating EVs in the plasma of AH patients compared to HD (particles/mL 9.3 × 1011 (IQR 4–24) versus 2.4 × 1011 (IQR 2–4), p = 0.03). The majority of the EVs identified in the two groups ranged in size from 40 to 200 nm, with an increased prevalence of larger and more size-disperse EVs in the plasma samples of AH patients when compared to HD (Supplementary Figure S2A,B). When observed by Cryo-electron microscopy (CryoEM), EVs had a characteristic spherical or cup-shaped morphology (Supplementary Figure S2D).
Plasma EVs surface was phenotyped by flow cytometry using a MACSPLEX EV kit IO (Miltenyi, Bergisch Gladbach, Germany), human with 37 surface antigens. Among them, 20 were differentially expressed between the two groups (Figure 1A, Supplementary Table S1). Thirteen antigens were significantly lower in EVs from AH patients. These included cell-specific antigens from leucocytes (CD45), T-cells (CD2, CD3, and CD25), monocyte-macrophages (CD44, CD40, CD209, and CD29) and platelets (CD41b and CD42a), and specific functional markers such as the integrin of monocytes-platelets (CD49e), cell adhesion molecule required for leukocyte transendothelial migration (CD31), and the antigen-presenting molecule (HLA-ABC). In AH patients, there was a significantly higher proportion of EVs enriched with seven endothelial cell antigens (CD105, CD146, and CD62p), followed by epithelial (CD133 and CD326), stem cell (SSEA-4), and monocyte-macrophage (CD14) lineages (Figure 1A). A correlation analysis of EV antigens revealed conserved positive associations between the two groups but disrupted negative correlations in AH, but not in HD (Figure 1B and Supplementary Figure S3).
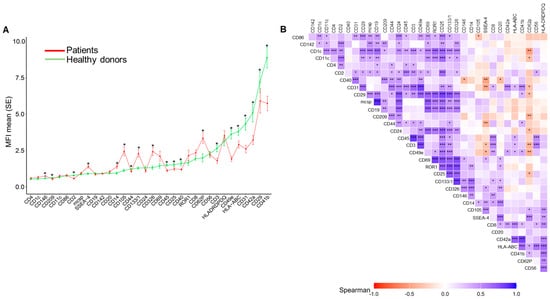
Figure 1.
Surface antigen profiles of extracellular vesicles (EVs) in healthy donors (HD) and alcohol-associated hepatitis (AH) patients: (A) Mean fluorescence intensity (MFI) profiles of 33 surface antigens expressed on EVs from HD (n= 28, green) and patients with AH (n = 46, red). Data are presented as mean ± standard deviation. Differences between groups were assessed using t-tests or Mann–Whitney tests, depending on the distribution of the values. (B) Correlation matrix of surface antigens expressed in EVs from AH patients. The heatmap displays pairwise Spearman correlation coefficients, with positive correlations shown in blue and negative correlations in red. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.2. EVs Surface Antigens Are Associated with the Main Clinical Features in the AH Episode
Figure 2 shows a graphical analysis that linked EVs surface antigens with clinical and epidemiological features in AH patients. The Alcohol Use Disorder Identification Test (AUDIT) score value, age, and drug use were associated with increased EVs from endothelial and progenitor cells and decreased EVs from immune cells. In contrast, metabolic risk factors (diabetes mellitus, body mass index [BMI], overweight, and obesity) correlated with increased EVs from platelets, endothelium, and monocytes. Specific blood cell counts also showed distinct correlations with EV profiles.
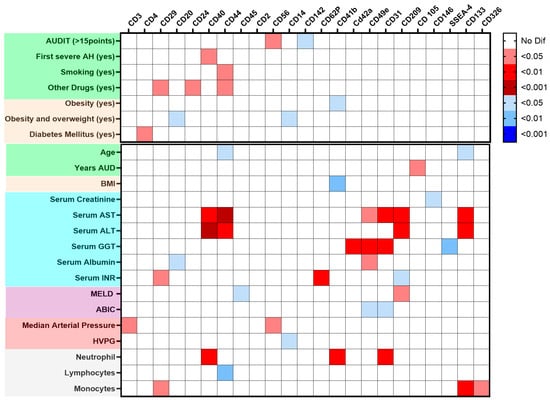
Figure 2.
Heatmap of the main extracellular vesicles (EVs), EV surface antigen, and the clinical data of alcohol-associated hepatitis (AH) patients. Data are presented as a heatmap built considering the p-value for p-values comparing the main EV surface antigens between patients (n = 46) and healthy donors HD (n = 28), along with qualitative and quantitative clinical characteristics at admission, colors on the left of both panels indicate feature types: green, age and toxic substance use; beige, weight and metabolic disorders; blue, biochemical values; purple, prognostic indices; red, hemodynamics parameters; and gray, blood cell counts. Top panel: Mann–Whitney tests were performed to evaluate differences in surface antigen expression in dichotomous clinical variables. The intensity represents the level of significance, with red indicating positive associations and blue indicating negative associations. Bottom panel: Correlation analysis between EV surface marker expression and continuous clinical variables. Spearman’s rank correlation coefficients were used, with red indicating positive correlations and blue indicating negative correlations.
Renal and liver function parameters showed distinct correlations with EV profiles. Creatinine levels were inversely correlated with leukocyte and EVs expressing endothelial markers. The transaminases aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were positively correlated with EVs expressing monocyte, endothelial, and progenitor cell markers, whereas gamma-glutamyl transferase (GGT) correlated with platelet EVs and EVs expressing endothelial markers. Total bilirubin and albumin were negatively associated with EVs expressing endothelial markers, whereas international normalized ratio (INR) was positively linked to endothelial EVs and negatively linked to EVs expressing monocyte markers. Hemodynamically, arterial pressure was positively associated with EVs expressing lymphocyte markers, and hepatic venous pressure gradient (HVPG) was inversely correlated with EVs expressing monocyte markers (Figure 2).
EVs’ surface antigens were correlated with liver function scores at admission (Supplementary Figure S4. The MELD score showed a positive correlation with EVs expressing monocyte markers (CD209) and a negative correlation with EVs expressing leukocyte markers (CD45). The ABIC score was inversely associated with monocyte-platelet integrin antigens (CD49e) and leukocyte transmigration (CD31). The Child–Pugh score negatively correlated with EVs expressing T cell (CD2, CD3), leukocyte (CD45), platelet integrin (CD49e), platelet antigen (CD42a), leukocyte transmigration (CD31), and activation/adhesion antigens (CD25, CD29).
In patients with AH, the most frequent complication during the index hospitalization was infection, occurring in 20 patients (43%). A total of 30 infectious episodes were identified, including bacterial pneumonia (6.21%), bacteremia (6.21%), urinary tract infection (6.21%), culture-negative spontaneous bacterial peritonitis (4.13%), esophageal candidiasis (2.7%), Clostridioides difficile colitis (2.7%), cellulitis (2.7%), and viral infections (2.7%). Another frequent complication was the AKI, observed in 12 patients (26%) (Supplementary Table S2).
Among patients who developed infections during hospitalization, we observed a higher proportion of EVs expressing B-cell markers (CD20) and a lower proportion expressing T-cell markers (CD2). In contrast, patients with AKI showed increased levels of EVs expressing monocyte antigen-presenting markers (CD40) and endothelial markers (CD146). In addition, several soluble inflammatory markers, including IL-6, sVCAM-1, sCD62P, and tumor necrosis factor receptor superfamily member 1 (TNFRSF1A), were correlated with EVs expressing monocyte and activated cell markers (CD14, CD25), as well as with EVs expressing endothelial markers (CD105 and CD146) (Figure 3).
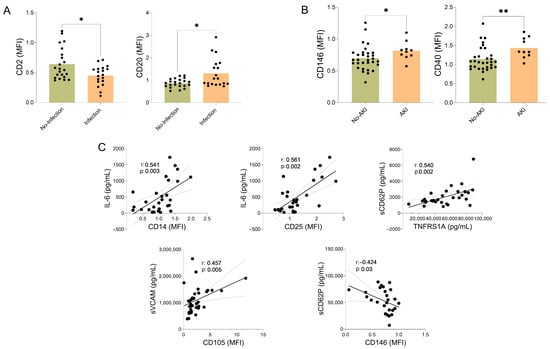
Figure 3.
Antigens on the surface of extracellular vesicles (EVs), infection, acute kidney injury (AKI), and soluble inflammatory mediators in AH patients. Association of EV surface antigens with infection, acute kidney injury (AKI), and soluble inflammatory mediators in AH patients. (A) Expression of CD2 and CD20 on plasma-derived EVs in AH patients with and without infections, data are presented as mean ± standard deviation. Differences between groups were assessed using t-tests or Mann–Whitney tests, depending on the distribution of the values; * p < 0.05. (B) Expression of CD146 and CD40 on plasma-derived EVs in AH patients with and without AKI, * p < 0.05, ** p < 0.01. The black dots indicate the values for each patient individually. (C) Correlation analysis between EV surface antigen expression and circulating soluble inflammatory mediators. Spearman’s correlation coefficient (r) and corresponding p-values are shown. The gray lines indicate the confidence intervals.
2.3. Diagnostic Performance of the EV Surface Antigen Associated with Corticosteroid Response and Long-Term Mortality
This study assessed whether EVs could be associated with corticosteroid response and survival in patients with AH. Among 46 patients, 34 received corticosteroids (74%), of which 21 were responders (62%) and 13 non-responders (38%) (Supplementary Table S3). Non-treatment with corticosteroid was primarily due to infections (43%) or rapid clinical improvement (42%). Responders were more frequently young, non-obese men. Compared to non-responders, they had less hyperdynamic circulation (lower free hepatic vein pressure and B-type natriuretic peptide (NT-ProBNP) and lower systemic inflammation (VCAM, TNFRSF1A), despite similar (C-reactive protein, IL-6), MELD score, and HVPG (Table 2).

Table 2.
Baseline demographic, clinical, and hemodynamic characteristics of responder versus non-responder patients to corticosteroid *.
Responders showed higher expression of EVs enriched with surface antigens CD49e and CD31, and lower levels of SSEA-4 and CD62p (Figure 4A).
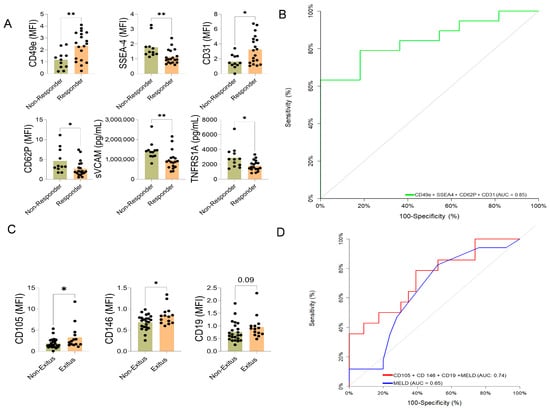
Figure 4.
Extracellular vesicle (EV) surface antigen associated with corticosteroid response and long-term mortality in AH patients. (A) EV surface antigens associated with corticosteroid response and long-term mortality in AH patients. Comparison of EV surface antigen cargo between patients who responded to steroid treatment (responders) and those who did not (non-responders). Statistically significant differences were calculated using a t-test or Mann–Whitney based on normal or non-normal distribution * p < 0.05, ** p < 0.005. The black dots indicate the values for each patient individually. (B) Combined multivariate predictive model of corticosteroid response based on EV surface antigen. The diagonal gray line represents a classifier with no discriminative power (i.e., random chance). (C) Comparison of EV surface antigen expression between patients who survived (survivors) and those who did not survive (non-survivors) at long-term follow-up. The black dots indicate the values for each patient individually. (D) Receiver operating characteristic curve (ROC) curve comparison between the MELD score (red line) and a custom multivariate model (blue line) incorporating EV surface antigen markers for predicting long-term mortality. The EVs-based model outperforms the MELD score in predictive accuracy. Statistically significant differences were calculated using a t-test or Mann–Whitney based on normal distribution or non-normal distribution * p < 0.05.
Given the observed clinical differences between responders and non-responders, the limited number of events (21 responders), and the associated risk of model overfitting, we performed multivariable analyses with age and sex as the only covariates. Under this conservative approach, the EVs markers remained independently associated with corticosteroid response, including CD49e (OR = 3.14; 95% CI, 1.19–12.20; p = 0.045), CD31 (OR = 1.91; 95% CI, 1.02–3.91; p = 0.042), SSEA4 (OR = 0.056; 95% CI, 0.003–0.48; p = 0.025), and CD62p (OR = 0.58; 95% CI, 0.31–0.87; p = 0.030). These findings suggest that the associations between EV markers and corticosteroid response are not solely explained by age or sex, although residual confounding by other clinical factors cannot be fully excluded.
The individual association of each EVs antigens was evaluated by area under the curve (AUC), and the final corticosteroid response model included CD49e, CD31, SSEA-4, and CD62p with an AUC 0.847 (95% CI: 0.71–0.98, p < 0.001), and with a net reclassification improvement (NRI) (1.22 CI: (0.63–1.80), p < 0.001)—integrated discrimination improvement (IDI) (0.33 CI: (0.15–0.51), p < 0.001) (Figure 4B).
In addition, we assessed whether EVs could be associated with mortality during the median follow-up of 1.7 (0.64–2.70) years. Fifty-four percent of patients survived, whereas 37% died or underwent liver transplantation (LT), and 9% were lost to follow-up. Patients who died or underwent transplantation had a higher BMI, a more pronounced hyperdynamic circulation—characterized by higher free hepatic venous pressure and lower mean arterial pressure—and elevated IL-6 levels. The main characteristics of these patients are summarized and compared in Table 3.

Table 3.
Baseline demographic, clinical, and hemodynamic characteristics of patients Alive versus Liver Transplanted (LT) or Dead at long-term follow-up **.
These differences suggest a more severe inflammatory and circulatory profile in patients who died or underwent LT (Supplementary Table S4). The EVs associated with death/LT during follow-up showed increased expression of the B-cell marker CD19 and the angiogenesis-related molecules endoglin (CD105) and MCAM (CD146) (Figure 4C). The AUC evaluated the individual capacity of each EVs antigens (CD19, CD105, and CD146), and the final model included CD19, CD105, CD146, and MELD score with an AUC: 0.74 ((95% CI: 0.57–0.91), p = 0.006) vs. MELD score alone AUC: 0.65 ((95% CI: 0.48–0.82), p = 0.09), with an NRI (0.34 CI:(0.30–0.97), p = 0.29)—IDI (0.19 CI:(0.04–0.34), p = 0.015) (Figure 4D).
2.4. Impact of Alcohol Consumption During the First 90 Days After AH Epidose on EVs Surface Antigen Expression
At the 90-day follow-up, 30 patients (65%) were assessed for alcohol use through the AUDIT questionnaire and Phosphatidylethanol (PEth) levels. Of these, 57% maintained abstinence, while 43% continued drinking. In patients who resumed alcohol consumption (13 patients), EVs were enriched by monocyte (CD14) and B cell (CD20) markers (Supplementary Figure S5).
3. Discussion
To the best of our knowledge, this is the first study to characterize the enrichment of surface antigens on plasma EVs in AH using multiplex flow cytometry. We identified a significant increase in circulating EVs, enriched in surface antigens of lymphocyte activation, endothelial injury, and hepatic progenitor cell involvement (Supplementary Table S1). These EVs were associated with key clinical outcomes, including corticosteroid response and mortality. Key EVs enriched with antigens linked to corticosteroid response may have clinical utility, especially for non-responders identified on day four. This approach may support the timely selection of patients for early LT evaluation.
The first significant finding of this study is the quantitative and qualitative characterization of EVs in AH. Compared with HD, AH patients exhibited increased numbers of EVs enriched for markers of immune cells, endothelial cells, and liver progenitor cells. Most of these antigens, particularly those linked to monocyte and lymphocyte activation, may reflect the immunotoxic effects of alcohol and the possible underlying immune dysregulation in the coordination of EVs production. Notably, patients who continued alcohol consumption at 90 days showed persistent alterations in EVs enriched with immune cell markers, suggesting immune activation associated with subsequent alcohol relapse (Supplementary Figure S5). The increase in plasma EV quantity observed in AH patients is consistent with previous studies [16,27,28]. In addition, the expression of specific surface markers suggests that these EVs, enriched with dysregulated immune cells and endothelial markers, may contribute to an increased risk of bacterial infections during the AH episode, impaired recovery, and mediate the poor prognosis.
Nevertheless, our findings are consistent with a clinical study suggesting that EVs from alcohol-exposed hepatocytes, carrying liver-specific miR-122, sensitize monocytes to lipopolysaccharides and amplify inflammation [29]. Our data are consistent with a potential role of EVs-related signaling in immune dysregulation in AH; however, these findings are exploratory and may reflect complex interactions involving intrahepatic monocyte activity, endothelial activation, and liver progenitor cell responses rather than direct causal mechanisms [21,32,33]. Moreover, because our analysis was limited to circulating EVs, the precise cell and tissue origins of these vesicles remain to be confirmed.
The second major finding of this study is an increase in EVs enriched with antigens from the adaptive immune system and endothelial activation, both of which are associated with the two main clinical complications of AH during hospitalization: infections and AKI. EVs enriched with adaptive immune cell markers (e.g., reduced CD2-T lymphocyte activation and increased CD20-B lymphocyte activation) were linked to in-hospital infections, which aligns with previous evidence indicating that, during infectious diseases, immune cell-derived EVs carry antigen-presenting and activation molecules that modulate host immune responses [34]. In contrast, those enriched in endothelial (CD146) and immune activation (CD40) markers were associated with AKI. In addition, we observed a significant increase in inflammatory markers (IL-6, TNFRS1A, and sVCAM), which may be involved in the SIRS often observed in patients with AH [3]. To the best of our knowledge, this is the first report describing an increase in EVs bearing CD40 and CD146 in the setting of AKI. CD146 has been identified as a key player in the pathogenesis of inflammatory diseases and as a potential diagnostic marker for acute kidney transplant rejection [35]. In liver disease, CD146 has also shown promise as a cost-effective surrogate biomarker for cirrhosis, with a good correlation with disease severity [36]. Similarly, CD40 has been implicated in models predicting renal outcomes after LT, acting as an inflammatory mediator potentially involved in immune-mediated renal injury [37]. Given that endothelial dysfunction and microvascular injury are central mechanisms contributing to AKI, we hypothesize that peripheral EVs enriched in surface antigens of endothelial damage (CD146) and immune activation (CD40) may act as mediators of liver–kidney crosstalk. These vesicles may contribute to processes linked to kidney injury in AH, potentially through pathways involving endothelial activation, leukocyte recruitment, and tubular damage; however, these observations should be considered exploratory.
The third key finding is the identification of a specific plasmatic EVs antigen profile associated with corticosteroid response and long-term mortality in patients with AH. In this study, we demonstrated that the EV antigen profile in plasma can distinguish responders from non-responders to corticosteroid therapy. These EVs features may reflect a more favorable immune and endothelial microenvironment that supports therapeutic responsiveness. Responders were young, non-obese men with higher levels of EVs enriched with antigens of endothelial activation and cell transmigration, along with lower levels of liver progenitor cell antigens and inflammatory signals. To date, only one previous study has demonstrated the predictive value of EVs in this setting, showing that elevated levels of CD34+ (endothelial cells) and asialoglycoprotein receptor 1 (ASGPR1+) (expressed on the sinusoidal surface of hepatocytes) microvesicles may serve as non-invasive markers of non-response to corticosteroid [31]. However, our findings suggest that higher levels of EVs enriched in CD31 and CD49e, together with lower expression of SSEA4 and CD62p in responders, may be associated with enhanced, more organized cell migration, improved endothelial barrier integrity, and potentially contribute to increased responsiveness to corticosteroid therapy. In this context, EVs enriched mainly in CD31 may be involved in an early, adaptive, and reparative response that facilitates immune cell trafficking and tissue repair, thereby being associated with corticosteroid responsiveness [38]. Collectively, these EV-associated antigens linked to corticosteroid response may have clinical utility as early markers, particularly for identifying non-responders as early as day 4 of therapy. This approach may help support the timely identification of patients who could benefit from early liver transplantation evaluation. Nevertheless, these exploratory results and the hypothesis-generating nature of our findings require confirmation in future functional and prospective studies.
In contrast, elevated EVs expressing CD105, CD146, and CD19 were associated with increased mortality, suggesting sustained endothelial activation and B-cell dysregulation as adverse prognostic features. Antigens associated with increased mortality included antigens of adaptive immune activation (CD19) and endothelial activation (CD105 and CD146), as well as higher systemic inflammation (IL-6). These antigens have been studied individually in the context of liver disease. Elevated levels of IL-6 have been associated with poorer outcomes across various liver diseases [39]. CD19+ B cells, particularly intrahepatic memory B and plasma cell subpopulations, are altered in acute-on-chronic liver failure [40]. While direct studies evaluating CD105 and CD146 in the context of AH are currently lacking, our findings suggest that persistent or excessive endothelial activation over time may contribute to endothelial dysfunction, microvascular thrombosis, and subsequent poor clinical outcomes and increased mortality. In this context, EVs enriched in CD105 (endoglin), a transforming growth factor-β (TGF-β) co-receptor expressed on endothelial cells and involved in angiogenesis and vascular remodeling [41,42], may reflect heightened endothelial activation and proliferation. CD105 is a well-recognized marker of activated, proliferative endothelium and has been linked to pathological angiogenesis and fibrosis, correlating with disease severity and increased mortality. Altogether, these findings suggest that elevated EVs enriched in CD105 may indicate endothelial dysfunction and maladaptive angiogenic responses in severe disease [42,43]. CD146, as previously mentioned, has been proposed as a marker for cirrhosis and correlates with disease severity [36]. In our study, it has a strong positive correlation with the ABIC score. All these molecules may be relevant in liver disease progression and outcomes in AH. These changes likely reflect underlying pathogenic processes, including maladaptive endothelial activation and microvascular injury. Further studies are required to determine the clinical utility of these EV-associated antigens for risk stratification and transplant decision-making.
In summary, our findings suggest that EVs enriched with endothelial activation antigens may initially reflect an adaptive, reparative response associated with corticosteroid responsiveness, whereas persistent or excessive activation may lead to endothelial dysfunction and maladaptive processes, contributing to microvascular injury, inflammation, and multi-organ failure.
This study has several limitations: (a) a relatively small sample size from patients with AH; (b) the limited number of female patients included (which is a common feature of AH cohorts) may have limited the detection of potential sex-specific differences; (c) the absence of a group of patients admitted with ALD (limited generalizability to other stages of alcohol-associated liver disease; (d) one hospital center; and (e) the tissue origin of circulating EVs have yet to be fully elucidated. EVs expressing endothelial or immune-associated surface markers may predominantly reflect contributions from the corresponding cell types, although alternative cellular sources cannot be excluded. The strengths are as follows: the prospective design, systematic sampling and clinical characterization, the use of multiplex EV profiling technology, and the confirmation of abstinence via PEth at 90 days.
In conclusion, our findings demonstrate the potential clinical utility of profiling EV surface antigens in patients with AH. EVs enriched with surface markers of activated endothelial cells, adaptive immune cells, and hepatic progenitor cells were associated with significant clinical outcomes. The main EVs enriched with antigens associated with corticosteroid response could be applied in clinical practice, particularly for the early identification of non-responders on day four. This approach may support the timely selection of patients for early LT evaluation. These observations should be considered exploratory and hypothesis-generating. Further translational and functional research, along with validation in larger, multicenter cohorts, are warranted to confirm these observations and explore their application in guiding therapeutic decisions.
4. Materials and Methods
4.1. Population and Study Design
Consecutive patients with a clinical suspicion of AH were prospectively enrolled at Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) between June 2020 and December 2024, before initiating corticosteroid treatment. All patients were followed prospectively until loss to follow-up, death, or LT. Clinical suspicion of AH was based on the following: excessive alcohol consumption (>60 g/day) before admission, moderately elevated aminotransferases (AST and ALT), high GGT, and serum bilirubin levels. Severe AH was defined as an Age, Bilirubin, INR, and Creatinine (ABIC) score ≥ 6.71 (ABIC B and C) or MEL > 20 points at admission, and histological confirmation was obtained for those patients on whom it was possible to perform a transjugular liver biopsy (TJLB) (36 patients) [9,44]. Psychiatric comorbidities were defined and classified according to DSM-5 [45]. The right cardiovascular pressures and HVPG were assessed according to the BAVENO VII guidelines [46].
Exclusion criteria were treatment with corticosteroids before TJLB, hepatocellular carcinoma, and active extra-hepatic cancer.
Blood Collection and Plasma Preparation
Peripheral blood was collected from 28 HD patients and from 46 AH patients at admission, using BD Vacutainer Citrate Tubes (BD Biosciences, San Jose, CA, USA). Platelet-poor plasma was obtained by centrifuging fresh whole blood at 1.000× g for 10 min. The resulting plasma was aliquoted and stored at −20 °C until analysis. According to the MISEV2018 guidelines and previous studies, EVs retain functional uptake characteristics after storage at −20 °C, supporting the suitability of this storage condition for EV analysis in clinical samples [24,25,47]. Blood cell counts and clinical and biochemical parameters were collected at baseline and at control times by hepatologists and the laboratory at Hospital de la Santa Creu i Sant Pau.
4.2. EVs Characterization
EVs’ size and concentration were assessed by NTA and visualized by Cryo-EM following MISEV 2024 guidelines [25].
4.2.1. Nanoparticle Tracking Analysis (NTA)
The concentration and size distribution of the EV preparations were determined by measuring Brownian motion using a NanoSight LM10 system equipped with fast video capture and particle-tracking software v2.3.5 (Malvern Instruments, Malvern, UK). Pre- and post-acquisition settings were maintained the same for all samples, and each video was analyzed to determine the particle size mode and estimate particle concentration. Each sample was acquired three times. Then, an average curve was calculated for each sample.
4.2.2. Cryo-Electron Microscopy
EVs preparations were directly adsorbed onto glow-discharged holey carbon grids R2/1 300 Mesh (QUANTIFOIL, Großlöbichau, Germany). Grids were blotted at 95% humidity and rapidly plunged into liquid ethane using a LEICA EM GP2 (Leica Microsystems, Wetzlar, Germany). Vitrified samples were imaged at liquid nitrogen temperature using a JEM1230 transmission cryo-electron microscope (JEOL, Tokyo Japan) equipped with a 120-kV LaB6 thermionic gun and with an Orius SC1000 (4008 × 2672 pixels) cooled slow-scan CCD camera (GATAN, Abingdon UK).
4.2.3. EVs Surface Antigen Analysis by Multiplex Bead-Based Technique
The plasma samples were evaluated by a multiplex bead-based EVs capture and analyzed by flow cytometry using a MACSPlex EV kit IO, human [48]. Plasma samples were incubated with the beads overnight. There were 37 fluorescently labeled capture bead populations, each coated with a specific antibody binding the respective surface antigen, and two control bead populations. The EVs attached to the beads were identified using allophycocyanin-conjugated antibodies against CD9, CD63, and CD81. Median fluorescence intensity (MFI) was measured on a MACSQuant Analyzer 10 flow cytometer (Miltenyi, Bergisch Gladbach, Germany) in line with previous validation studies [49].
The assay was technically reproducible, and all final results were based on plasma-derived EVs. The stored plasma samples were processed using two consecutive centrifugations to further purify the plasma (30 min at 2000× g, 45 min at 10,000× g). A total of 30 µL of centrifuged plasma samples was diluted by 1/5 using the buffer solution, and the final volume added to each well of the kit was 130 µL of diluted plasma, which was analyzed. We used MacsPlex Buffer incubated with beads and detection antibodies as a blank control. All analyses were performed using normalized MFI (nMFI) values, calculated as the MFI of the individual marker divided by the MFI of its immunoglobulin isotype control, and sample evaluations were performed without knowledge of the clinical diagnosis. All antigens were analyzed simultaneously.
4.3. Determination of Soluble Pro-Inflammatory Mediators
Plasma concentrations of interleukin-6 (IL-6; catalog no. 3460-1H-6, Mabtech, Nacka Strand, Sweden), TNF receptor superfamily member 1A (TNFRSF1A; catalog no. DY225-05), vascular cell adhesion molecule-1 (VCAM-1; catalog no. DY809-05), and intercellular adhesion molecule-1 (ICAM-1; catalog no. DY720) were measured in patients with AH using commercially available ELISA kits and according to the manufacturers instructions (all from R&D Systems, Minneapolis, MN, USA).
4.4. Statistical Analysis
Normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables were expressed as mean (standard deviation) or median (interquartile range) as appropriate. Differences in continuous variables were tested by Student’s t-test, Mann–Whitney tests or the Wilcoxon signed rank test, considering independent or related samples as applicable. Categorical variables were presented as frequency and percentage. Differences in the categorical variables were assessed by the χ2 test or by Fisher’s exact test. Correlation between the Es antigens and quantitative variables was assessed using Spearman’s correlation. p < 0.05 were considered statistically significant. The association between the corticosteroid response and different EVs was analyzed using age- and sex-adjusted logistic regression models. Given their clinical relevance, the limited number of events, and the associated risk of model overfitting, the number of covariates included in multivariable analyses was intentionally restricted. To evaluate the predictive potential of EVs, antigens associated with clinical outcomes, such as corticosteroid response and survival, with p-values less than 0.1 in the initial descriptive analysis were selected for further assessment using the Area Under the Curve (AUC) and the DeLong test. Building upon a neutral baseline model, we sequentially examined the additional prognostic significance of each antigen and its combinations by measuring improvements in AUC, integrated discrimination improvement (IDI), and net reclassification improvement (NRI) [50]. To compare the progression of continuous variables between the two groups, linear mixed models were employed. All statistical analyses were conducted using R software (v.4.4) (R Foundation for Statistical Computing, Vienna, Austria), SPSS Statistics (v.29) (IBM Corp., Armonk, NY, USA), and GraphPad Prism (10.2.3) (GraphPad Software, San Diego, CA, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms27031258/s1.
Author Contributions
Conceptualization, A.G.-C., A.B., R.O.-G., E.C., M.M., S.V. and E.A.-T.; Recruitment of patients, E.A.-T., A.B., M.C., J.F., M.P., R.G., J.V., À.E., E.S.-A., E.R. and C.G.; liver biopsy and hemodynamic study, A.B., E.A.-T., J.F., R.G. and J.V.; samples obtention and processing, E.R., C.G. and E.S.-A.; quantification and characterization of extracellular vesicles, A.G.-C., E.G. and J.M.F.-P.; psychological evaluation, F.D.; data curation, A.G.-C., A.B. and M.C.; formal analysis: A.G.-C., A.B., E.A.-T. and A.F.-G.; funding acquisition, E.A.-T. investigation A.G.-C., A.B., R.O.-G. and E.A.-T. methodology, A.G.-C., R.O.-G., E.C., M.M., E.A.-T. and S.V. project administration, S.V. and E.A.-T. resources, R.G., F.D., J.V., M.P., E.R., J.F., E.G., M.C., G.S., À.E., C.G., A.B., J.S., E.S.-A., S.V. and E.A.-T.; software, A.F.-G.; supervision, S.V. and E.A.-T.; visualization, A.G.-C., A.B. and E.A.-T.; writing—original draft, A.G.-C., A.B. and E.A.-T.; writing—review and editing, R.O.-G., G.S., J.F., R.B., J.A., S.V. and E.A.-T. All authors have read and agreed to the published version of the manuscript.
Funding
Edilmar Alvarado-Tapias is a recipient of a “Río Hortega” fellowship grant from the Instituto de Salud Carlos III (CM16/00133), a “Juan Rodés” post-doctoral grant from the Instituto de Salud Carlos III (JR20/00047), and the PI21/01995 grant from the ISCIII, FONDOS FEDER. EURO-VALDI-NET COST Action CA -23146 Short Term Scientific Mission (STSM) Grant 2025.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Institut de recerca Sant Pau (protocol code IIBSP-EHA-2021-132, on 15 March 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author.
Conflicts of Interest
R.B. has served as a consultant for GlaxoSmithKline (GSK), Novo Nordisk, and Boehringer Ingelheim. The remaining authors declare no conflicts of interest related to this work. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ABIC | Age-bilirubin-international normalized ratio-creatinine |
| AH | Alcohol-associated hepatitis |
| AKI | Acute Kidney injury |
| ALD | Alcohol-associated liver disease |
| ASGPR1 | Asialoglycoprotein |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| AUC | Area under the curve |
| AUD | Alcohol use disorder |
| AUDIT | Alcohol use disorders identification test |
| BMI | Body mass index |
| CD146 | Cluster of differentiation 146 (also known as MCAM) |
| DAMPs | Damage-associated molecular patterns |
| EVs | Extracellular vesicles |
| GGT | Gamma-glutamyl transpeptidase |
| HD | Healthy donors |
| HVPG | Hepatic venous pressure gradient |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IDI | Integrated discrimination improvement |
| IL-6 | Interleukin-6 |
| LT | Liver transplantation |
| MELD | Model for End-Stage Liver Disease |
| MFI | Median fluorescence intensity |
| NRI | Net reclassification improvement |
| NTA | Nanoparticle tracking analysis |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| PEth | Phosphatidylethanol |
| S | soluble |
| SEC | Size-exclusion chromatography |
| SIRS | Systemic inflammatory response syndrome |
| TJLB | Transjugular liver biopsy |
| TNFRS1A | Tumor necrosis factor receptor superfamily member 1A |
| VCAM | Vascular cell adhesion molecule 1 |
| VEGF-A | Vascular endothelial growth factor A |
References
- Stein, E.; Cruz-Lemini, M.; Altamirano, J.; Ndugga, N.; Couper, D.; Abraldes, J.G.; Bataller, R. Heavy Daily Alcohol Intake at the Population Level Predicts the Weight of Alcohol in Cirrhosis Burden Worldwide. J. Hepatol. 2016, 65, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Alcohol Collaborators. Alcohol Use and Burden for 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affò, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; García-Pagán, J.C.; Fernández, J.; Arroyo, V. Systemic Inflammatory Response and Serum Lipopolysaccharide Levels Predict Multiple Organ Failure and Death in Alcoholic Hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Gripshover, T.C.; Treves, R.S.; Rouchka, E.C.; Chariker, J.H.; Zheng, S.; Hudson, E.; Smith, M.L.; Singal, A.K.; McClain, C.J.; Hardesty, J.E. Visium Spatial Transcriptomics and Proteomics Identifies Novel Hepatic Cell Populations and Transcriptomic Signatures of Alcohol-associated Hepatitis. Alcohol Clin. Exp. Res. 2025, 49, 106–116. [Google Scholar] [CrossRef]
- Zuluaga, P.; Teniente-Serra, A.; Fuster, D.; Quirant-Sánchez, B.; Hernandez-Rubio, A.; Martínez-Cáceres, E.; Muga, R. Increased Natural Killer Cells Are Associated with Alcohol Liver Fibrosis and with T Cell and Cytotoxic Subpopulations Change. J. Clin. Med. 2022, 11, 305. [Google Scholar] [CrossRef]
- Louvet, A.; Wartel, F.; Castel, H.; Dharancy, S.; Hollebecque, A.; Canva–Delcambre, V.; Deltenre, P.; Mathurin, P. Infection in Patients with Severe Alcoholic Hepatitis Treated with Steroids: Early Response to Therapy Is the Key Factor. Gastroenterology 2009, 137, 541–548. [Google Scholar] [CrossRef]
- Ma, A.T.; Allegretti, A.S.; Cullaro, G.; Ouyang, T.; Asrani, S.K.; Chung, R.T.; Przybyszewski, E.M.; Wilechansky, R.M.; Robinson, J.E.; Sharma, P. Outcomes of Patients with Alcohol-associated Hepatitis and Acute Kidney Injury–Results from the HRS Harmony Consortium. Aliment. Pharmacol. Ther. 2024, 60, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA 2021, 326, 165–176. [Google Scholar] [CrossRef]
- Bataller, R.; Arab, J.P.; Shah, V.H. Alcohol-Associated Hepatitis. N. Engl. J. Med. 2022, 387, 2436–2448. [Google Scholar] [CrossRef]
- Altamirano, J.; López-Pelayo, H.; Michelena, J.; Jones, P.D.; Ortega, L.; Ginès, P.; Caballería, J.; Gual, A.; Bataller, R.; Lligoña, A. Alcohol Abstinence in Patients Surviving an Episode of Alcoholic Hepatitis: Prediction and Impact on Long-Term Survival. Hepatology 2017, 66, 1842–1853. [Google Scholar] [CrossRef]
- Mathurin, P.; O’Grady, J.; Carithers, R.L.; Phillips, M.; Louvet, A.; Mendenhall, C.L.; Ramond, M.-J.; Naveau, S.; Maddrey, W.C.; Morgan, T.R. Corticosteroids Improve Short-Term Survival in Patients with Severe Alcoholic Hepatitis: Meta-Analysis of Individual Patient Data. Gut 2011, 60, 255–260. [Google Scholar] [CrossRef]
- Louvet, A.; Thursz, M.R.; Kim, D.J.; Labreuche, J.; Atkinson, S.R.; Sidhu, S.S.; O’Grady, J.G.; Akriviadis, E.; Sinakos, E.; Carithers, R.L.; et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-Analysis of Individual Data From Controlled Trials. Gastroenterology 2018, 155, 458–468.e8. [Google Scholar] [CrossRef]
- Thursz, M.R.; Richardson, P.; Allison, M.; Austin, A.; Bowers, M.; Day, C.P.; Downs, N.; Gleeson, D.; MacGilchrist, A.; Grant, A.; et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N. Engl. J. Med. 2015, 372, 1619–1628. [Google Scholar] [CrossRef]
- Hmoud, B.S.; Patel, K.; Bataller, R.; Singal, A.K. Corticosteroids and Occurrence of and Mortality from Infections in Severe Alcoholic Hepatitis: A Meta-Analysis of Randomized Trials. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 721–728. [Google Scholar] [CrossRef]
- Vergis, N.; Atkinson, S.R.; Knapp, S.; Maurice, J.; Allison, M.; Austin, A.; Forrest, E.H.; Masson, S.; McCune, A.; Patch, D.; et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017, 152, 1068–1077.e4. [Google Scholar] [CrossRef]
- Bissonnette, J.; Altamirano, J.; Devue, C.; Roux, O.; Payancé, A.; Lebrec, D.; Bedossa, P.; Valla, D.; Durand, F.; Ait-Oufella, H.; et al. A Prospective Study of the Utility of Plasma Biomarkers to Diagnose Alcoholic Hepatitis. Hepatology 2017, 66, 555–563. [Google Scholar] [CrossRef]
- Atkinson, S.R.; Grove, J.I.; Liebig, S.; Astbury, S.; Vergis, N.; Goldin, R.; Quaglia, A.; Bantel, H.; Guha, I.N.; Thursz, M.R.; et al. In Severe Alcoholic Hepatitis, Serum Keratin-18 Fragments Are Diagnostic, Prognostic, and Theragnostic Biomarkers. Am. J. Gastroenterol. 2020, 115, 1857–1868. [Google Scholar] [CrossRef]
- Arab, J.P.; Díaz, L.A.; Baeza, N.; Idalsoaga, F.; Fuentes-López, E.; Arnold, J.; Ramírez, C.A.; Morales-Arraez, D.; Ventura-Cots, M.; Alvarado-Tapias, E.; et al. Identification of Optimal Therapeutic Window for Steroid Use in Severe Alcohol-Associated Hepatitis: A Worldwide Study. J. Hepatol. 2021, 75, 1026–1033. [Google Scholar] [CrossRef]
- Alvarado-Tapias, E.; Pose, E.; Gratacós-Ginès, J.; Clemente-Sánchez, A.; López-Pelayo, H.; Bataller, R. Alcohol-Associated Liver Disease: Natural History, Management and Novel Targeted Therapies. Clin. Mol. Hepatol. 2024, 31, S112–S133. [Google Scholar] [CrossRef]
- Wu, T.; Shah, V. Biomarkers of Endothelial Dysfunction in Alcoholic Hepatitis. Hepatol. Int. 2021, 15, 855–857. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, J.; Sanyal, A.J.; Shah, V.H.; Chalasani, N.P.; Yu, Q.; Zheng, X.; Li, W. Persistent Hyperactivation of Endothelial Cells in Patients with Alcoholic Hepatitis. Alcohol. Clin. Exp. Res. 2020, 44, 1075–1087. [Google Scholar] [CrossRef]
- Thietart, S.; Rautou, P.-E. Extracellular Vesicles as Biomarkers in Liver Diseases: A Clinician’s Point of View. J. Hepatol. 2020, 73, 1507–1525. [Google Scholar] [CrossRef]
- Kostallari, E.; Valainathan, S.; Biquard, L.; Shah, V.H.; Rautou, P.-E. Role of Extracellular Vesicles in Liver Diseases and Their Therapeutic Potential. Adv. Drug Deliv. Rev. 2021, 175, 113816. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.; Erdbrügger, U. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Hong, C.-S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of Biologically Active and Morphologically Intact Exosomes from Plasma of Patients with Cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Malhi, H. Emerging Role of Extracellular Vesicles in Liver Diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G739–G749. [Google Scholar] [CrossRef]
- Sehrawat, T.S.; Arab, J.P.; Liu, M.; Amrollahi, P.; Wan, M.; Fan, J.; Nakao, Y.; Pose, E.; Navarro-Corcuera, A.; Dasgupta, D.; et al. Circulating Extracellular Vesicles Carrying Sphingolipid Cargo for the Diagnosis and Dynamic Risk Profiling of Alcoholic Hepatitis. Hepatology 2021, 73, 571–585. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes Derived from Alcohol-Treated Hepatocytes Horizontally Transfer Liver Specific miRNA-122 and Sensitize Monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef]
- Eguchi, A.; Yan, R.; Pan, S.Q.; Wu, R.; Kim, J.; Chen, Y.; Ansong, C.; Smith, R.D.; Tempaku, M.; Ohno-Machado, L.; et al. Comprehensive Characterization of Hepatocyte-Derived Extracellular Vesicles Identifies Direct miRNA-Based Regulation of Hepatic Stellate Cells and DAMP-Based Hepatic Macrophage IL-1β and IL-17 Upregulation in Alcoholic Hepatitis Mice. J. Mol. Med. Berl. Ger. 2020, 98, 1021–1034. [Google Scholar] [CrossRef]
- Sukriti, S.; Maras, J.S.; Bihari, C.; Das, S.; Vyas, A.K.; Sharma, S.; Hussain, S.; Shasthry, S.; Choudhary, A.; Premkumar, M.; et al. Microvesicles in Hepatic and Peripheral Vein Can Predict Nonresponse to Corticosteroid Therapy in Severe Alcoholic Hepatitis. Aliment. Pharmacol. Ther. 2018, 47, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Virovic-Jukic, L.; Ljubas, D.; Stojsavljevic-Shapeski, S.; Ljubičić, N.; Kanizaj, T.F.; Mikolasevic, I.; Grgurevic, I. Liver Regeneration as Treatment Target for Severe Alcoholic Hepatitis. World J. Gastroenterol. 2022, 28, 4557. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Wu, X.; Allende, D.S.; Nagy, L.E. Gene Deconvolution Reveals Aberrant Liver Regeneration and Immune Cell Infiltration in Alcohol-associated Hepatitis. Hepatology 2021, 74, 987–1002. [Google Scholar] [CrossRef]
- Rodrigues, M.; Fan, J.; Lyon, C.; Wan, M.; Hu, Y. Role of Extracellular Vesicles in Viral and Bacterial Infections: Pathogenesis, Diagnostics, and Therapeutics. Theranostics 2018, 8, 2709. [Google Scholar] [CrossRef]
- Liao, J.; Fu, Q.; Chen, W.; Li, J.; Zhang, W.; Zhang, H.; Gao, Y.; Yang, S.; Xu, B.; Huang, H. Plasma Soluble CD146 as a Potential Diagnostic Marker of Acute Rejection in Kidney Transplantation. Front. Med. 2020, 7, 531999. [Google Scholar] [CrossRef]
- Nomikou, E.; Alexopoulou, A.; Vasilieva, L.; Agiasotelli, D.; Pavlou, E.; Theodossiades, G.; Dourakis, S.P. Soluble CD146, a Novel Endothelial Marker, Is Related to the Severity of Liver Disease. Scand. J. Gastroenterol. 2015, 50, 577–583. [Google Scholar] [CrossRef]
- Levitsky, J.; Asrani, S.K.; Klintmalm, G.; Schiano, T.; Moss, A.; Chavin, K.; Miller, C.; Guo, K.; Zhao, L.; Jennings, L.W. Discovery and Validation of a Biomarker Model (PRESERVE) Predictive of Renal Outcomes after Liver Transplantation. Hepatology 2020, 71, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, J.; Hawthorne, M.; Day, L.; Warner, J.; Warner, D.; Gritsenko, M.; Asghar, A.; Stolz, A.; Morgan, T.; McClain, C. Steroid Responsiveness in Alcohol-Associated Hepatitis Is Linked to Glucocorticoid Metabolism, Mitochondrial Repair, and Heat Shock Proteins. Hepatol. Commun. 2024, 8, e0393. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, N.; He, T.-T.; Wang, Y.; Wang, L.-F.; Sun, Y.-Q.; Jing, J.; Zhang, J.-J.; Fu, S.-N.; Wang, X. High Levels of Serum Interleukin-6 Increase Mortality of Hepatitis B Virus-Associated Acute-on-Chronic Liver Failure. World J. Gastroenterol. 2020, 26, 4479. [Google Scholar] [CrossRef]
- Zhao, Y.; He, W.; Wang, C.; Cui, N.; Yang, C.; You, Z.; Shi, B.; Xia, L.; Chen, X. Characterization of Intrahepatic B Cells in Acute-on-Chronic Liver Failure. Front. Immunol. 2022, 13, 1041176, Corrigendum in Front. Immunol. 2025, 16, 1581969. [Google Scholar] [CrossRef]
- Rossi, E.; Bernabeu, C.; Smadja, D.M. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function beyond TGF-β. Front. Med. 2019, 6, 10. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887. [Google Scholar] [CrossRef]
- Meurer, S.K.; Alsamman, M.; Scholten, D.; Weiskirchen, R. Endoglin in Liver Fibrogenesis: Bridging Basic Science and Clinical Practice. World J. Biol. Chem. 2014, 5, 180. [Google Scholar] [CrossRef]
- Dominguez, M.; Rincón, D.; Abraldes, J.G.; Miquel, R.; Colmenero, J.; Bellot, P.; García-Pagán, J.-C.; Fernández, R.; Moreno, M.; Bañares, R. A New Scoring System for Prognostic Stratification of Patients with Alcoholic Hepatitis. Off. J. Am. Coll. Gastroenterol. ACG 2008, 103, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R. Baveno VII–Renewing Consensus in Portal Hypertension. J. Hepatol. 2022, 76, 959–974, Erratum in J. Hepatol. 2022, 77, 271. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Bianco, G.; Burrello, A.; Manno, C.; Maulucci, F.; Pileggi, M.; Nannoni, S.; Michel, P.; Bolis, S.; Melli, G. Extracellular Vesicle Surface Markers as a Diagnostic Tool in Transient Ischemic Attacks. Stroke 2021, 52, 3335–3347. [Google Scholar] [CrossRef]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.; Bosio, A.; Schauss, A.; Wild, S. A Novel Multiplex Bead-Based Platform Highlights the Diversity of Extracellular Vesicles. J. Extracell. Vesicles 2016, 5, 29975. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the Added Predictive Ability of a New Marker: From Area under the ROC Curve to Reclassification and Beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.