Abstract
Targeted synthesis of Ni/C-containing composite materials was carried out using the matrix isolation method. The Ni content was varied (5–20 wt.% from chitosan). The morphology and physicochemical properties of the obtained materials were characterized using a number of methods: elemental analysis, SEM, TEM, XRD, FTIR, Raman spectroscopy, TPR–H2, and SSA. FTIR showed that nickel ions are immobilized on the chitosan molecule, and heat treatment of the polymer molecule results in the formation of polyconjugation centers. It was also revealed that heat treatment of the salt–polymer films results in the formation of a graphite-like structure (Raman spectroscopy) with the inclusion of nickel in metallic form (XRD, TPR–H2), with a particle size from 2 to 10 nm (TEM). The composites were shown to have a SSA of up to 269 m2/g. The resulting composite materials were used as catalysts for the decomposition of methane to produce hydrogen. High activity was observed in the catalytic methane decomposition at 700 °C (methane conversion up to 25.8%; hydrogen yield up to 1.98 gH2/gNi/h).
1. Introduction
The field of nanotechnology and nanomaterial synthesis is currently actively developing, making it possible to take into account and balance certain economic and social aspects. The introduction of nanotechnology has helped improve such fundamental areas of industry as electronics and information systems, medicine, and biological sciences, as well as energy and environmental safety [1]. This field is a relevant topic at the present time [2,3,4]. In this regard, the synthesis of nanoscale materials is a key priority in the development of modern science. An analysis of current trends in the field of nanomaterials reveals two of the most pressing challenges faced by scientists: the development of a methodology for obtaining new nanomaterials and a comprehensive study of their physicochemical properties, followed by the determination of their areas of application [5,6,7].
One of the key areas where the solution and improvement of these problems could lead to significant progress is heterogeneous catalysis. Materials for heterogeneous catalysis, despite many advantages, namely, mechanical strength, chemical stability, and ease of preparation, are inferior to homogeneous materials in the main process parameters, for example, in selectivity and conversion. The presence of these disadvantages necessitates the development of new heterogeneous materials [8,9,10,11]. An important criterion for the creation of new materials is the selection of the most effective support. Metal oxides, zeolites, and carbon supports are mainly used as a base [12,13]. The choice in favor of these systems is determined by their high chemical and thermal stability.
Recently, research using polymers with immobilized metal nanoparticles as carriers has been actively developing [14,15,16,17]. For example, in the work of Wissing M. et al. [18], gold and palladium nanoparticles on zeolite, combined with photoactive polymer brushes, were developed. In the work of Nagarjuna R. et al. [19], a Pd-containing catalyst was synthesized using polyethyleneimine based on Al2O3 with subsequent adsorption of palladium.
Scientific interest is currently shifting towards the search for and development of environmentally friendly and renewable alternatives that will be able to replace classical synthetic polymers. One such area of modern research, which meets the principles of green chemistry, is the creation of safe nanomaterials from renewable sources [20]. Taking into account all the requirements for safe materials, natural polysaccharides are promising in this area. Due to the diversity of reactive functional groups in their structure, polysaccharides are capable of forming strong composite materials with various inorganic materials and metal ions [21,22,23]. Thus, in the work of de Almeida D.A. et al. [24], scientists synthesized hydrogel composites based on pectin with gold nanoparticles. In the work [25], palladium nanoparticles stabilized with gelatin/pectin were obtained as materials.
Among the wide variety of polysaccharides, chitosan demonstrates the greatest potential in the creation of nanocomposite materials. Chitosan, as a natural polysaccharide, attracts attention due to the combination of several properties: high chemical activity, non-toxicity, renewableness, and availability [26]. Chitosan is studied as a complexing and stabilizing agent in the synthesis of catalytic systems [27]. Due to the presence of active amino groups, thermal stability, and inertness in organic media, chitosan is a multifunctional matrix for the immobilization of metal-containing nanoparticles intended for use in catalysis. The incorporation of metal nanoparticles into the polymer matrices of composite materials can improve the properties of these materials, namely, increase chemical and thermal resistance and provide higher mechanical properties [28,29]. Metal/polymer composites can be obtained by matrix isolation, where the polymer acts as a stabilizer for metal nanoparticles. This method allows for preventing the coagulation of metal nanoparticles while maintaining their properties [30].
When selecting an active metal for immobilization in a polymer matrix, transition metals are of particular interest. Group VIII metals are often used in gas chemistry processes due to the presence of partially filled 3 d-electron orbitals that are capable of accepting additional electrons. Thus, in [31], a series of nano-catalysts with the composition Fe5–Co–Zn/Al2O3 with varying stoichiometric coefficients of Co and Zn were synthesized for the CH4 decomposition process. In another study [32], bimetallic MoNi nanoparticles supported on MgO were also investigated in the methane decomposition process. The use of nickel as an active metal is the most promising since it is more reactive than Fe and Co and operates at lower temperatures in the methane decomposition process [33,34]. In addition to the metals listed above, other elements capable of immobilization on carbon supports can also serve as active components. For example, Ref. [35] described a photothermal catalyst based on a stable and uniformly dispersed graphene-like biomaterial, in the matrix of which atomically dispersed neighboring potassium atoms are immobilized. The resulting system has been successfully used for the catalytic production of biodiesel fuel. Another study [36] presented a highly efficient Bi1−xVO4—Cu catalytic system, where copper atoms are fixed to bismuth vacancies in the BiVO4 structure. This catalyst demonstrates exceptional activity in the electrocatalytic synthesis of methane from CO2 with a high kinetic isotope effect and high specific productivity.
Today, there are many environmental problems in the world, one of which is the emission of greenhouse gases [37,38]. To reduce environmentally harmful COx emissions, a method for catalytic decomposition of methane (CDM) was developed, which allows for obtaining “turquoise” hydrogen and carbon in solid form. The classic temperature of methane decomposition is 1200 °C, while in CDM, the temperature is 700–800 °C [39,40,41]. Catalysts for the catalytic decomposition of methane are divided into two main groups: metal-containing and carbon materials. One of their main differences is the temperature regime: carbon materials are active from 800 °C, while metal-containing systems are active in a lower range of 600–700 °C [42,43]. Carbon materials, in turn, have high stability, which is due to the developed specific surface area. Metal-containing catalysts, despite high activity, are subject to deactivation due to carbonization, which negatively affects the process [44,45]. To prevent these disadvantages, attempts are being made to combine metal-containing and carbon materials that will combine a developed specific surface area and high activity.
In this study, composite materials based on chitosan with added nickel were synthesized for the first time using matrix isolation in an inert atmosphere and characterized. The catalytic activity of the resulting Ni-containing carbon composites was studied in the methane decomposition reaction.
2. Results and Discussion
2.1. Characteristics of Materials
2.1.1. Elemental Analysis
Table 1 presents a list of the resulting composites and their elemental composition. The nickel content was varied across the catalyst line. To achieve this, the amount of nickel nitrate was increased, and the Ni/chitosan ratio was varied as follows: 1/20 → 2/20 → 3/20 → 4/20, corresponding to Ni contents of Ni 5, 10, 15, and 20 wt.% from chitosan. After heat treatment of the catalyst precursors, an increase in the Ni content relative to the initial one (~2 times) is observed. The highest nickel content is characteristic of the Ni/Ch–4 sample, which reaches 47.5 wt.%. Also, with an increase in the nickel content in the composites, a decrease in the content of carbon (from 61.7 to 40.7 wt.%), nitrogen (from 11.3 to 6.7 wt.%), and hydrogen (from 2.1 to 1.1 wt.%) is observed.

Table 1.
Elemental analysis of composite materials.
2.1.2. X-Ray
The X-ray diffraction patterns of the composite materials are shown in Figure 1. During heat treatment, all samples were characterized by the formation of the Ni phase (PDF#65–2865, 2θ = 44.5°, 51.8°, 76.4°, 92.9°, 98.4°). Also, for the Ni/Ch–3 and Ni/Ch–4 samples, the appearance of the NiO phase can be judged by the following low-intensity peaks 2θ = 37.5°; 41.8°; 62.8°; 79.0° (PDF#89–7130). The stability of the Ni phase formed during the catalyst synthesis is possibly due to the fact that most of the metal is covered with carbon. And the presence of the NiO phase in the Ni/Ch–3 and Ni/Ch–4 samples is probably due to the insufficient amount of polymer destruction products for the complete reduction of NiO to Ni. The low intensity of the NiO phase reflections is likely due to either the X-ray amorphous nature of this phase or the excessively small particle size. The X-ray diffraction results also suggest the formation of a highly dispersed system. The average nickel crystallite size was 4–6 nm for all catalyst samples except Ni/Ch–4, for which the Ni crystallite size was 15 nm, likely due to the high thermal mobility of the metal.
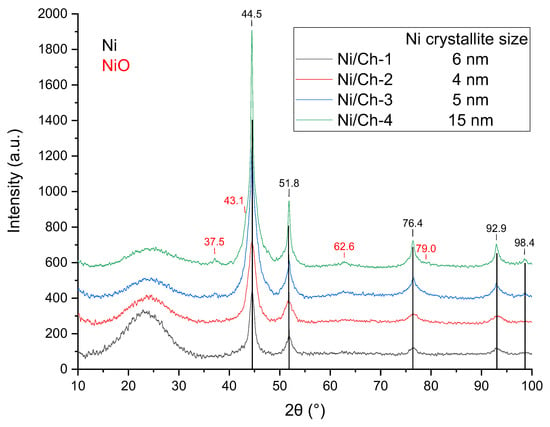
Figure 1.
XRD of composite materials.
2.1.3. TPR–H2
The TPR–H2 spectra for the catalysts are shown in Figure 2. According to the TPR–H2 data for the Ni/Ch–3 and Ni/Ch–4 composite material samples, two main reduction peaks are observed for the Ni/Ch–3 and Ni/Ch–4 composite material samples, one in the temperature range of 75–200 °C and the other with a maximum at about 500 °C. The first reduction peak is due to hydrogen absorption by the carbon matrix, which results in the removal of oxygen and nitrogen atoms from the catalyst. The peaks in the 500 °C region correspond to the temperature range of NiO reduction to metallic nickel (Ni2+ → Ni0). Moreover, the amount of absorbed hydrogen for the Ni/Ch–4 sample is 1.8 times greater than for Ni/Ch–3. However, for the Ni/Ch–1 and Ni/Ch–2 catalyst samples, virtually no hydrogen absorption is observed in the 500 °C region, which is likely due to the fairly complete reduction of NiO during the catalyst synthesis. It can be concluded that the released H2 and CO, when exposed to thermal stress on the metal–polymer film, reduce NiO to metallic nickel (Ni2+ → Ni0), but with an increase in the NiO content in the precursor, H2 and CO are not enough for reduction, and some of the NiO remains after thermal stress, which is consistent with the XRD results (Figure 1).
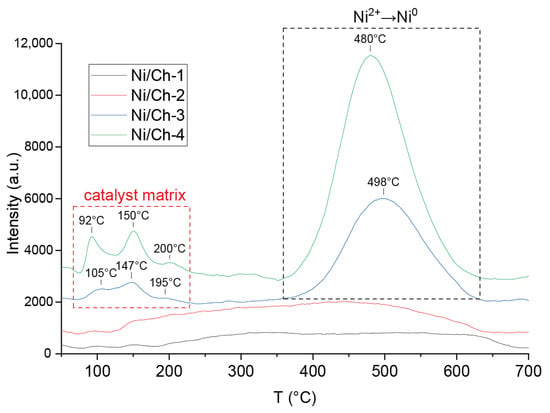
Figure 2.
TPR–H2 profile of samples of composite materials.
2.1.4. IR Spectroscopy
Figure S1 (Supplementary Materials) shows the IR spectra of the initial chitosan and the salt–polymer films, and Table 2 presents the wavenumbers of the main bands. The spectrum of chitosan is similar to the spectra presented in [46,47,48]. In the IR spectrum of chitosan, a broad band with a maximum at 3366 cm−1 corresponds to the stretching vibrations of –NH2 and –OH. For the salt–polymer films, a shift in this band to the region of lower frequencies (~3310 cm−1) is observed, which indicates that the –NH2 and –OH groups are involved in complexation [49]. The appearance of a new peak in the region of 3177–3180 cm−1, the intensity of which increases with increasing nickel content, can also be associated with the redistribution of hydrogen bonds due to complexation. The wavenumbers of the chitosan bands with maxima at 2914 and 2874 cm−1 correspond to the –CH2 stretching vibrations in the pyranose ring. With the addition of Ni(NO3)2, the wavenumbers related to the –CH2 stretching vibrations change only slightly. The formation of a chelate complex is also indirectly confirmed by the absorption bands corresponding to the amide bands. Thus, with the introduction of a nickel salt into the polymer, a shift in the C=O (amide I) absorption band to longer wavelengths (from 1653 to 1601 cm−1) is observed. A similar shift in the absorption band is characteristic of NH deformation vibrations. The appearance of absorption bands with maxima in the range of 1630–1600 cm−1 and 1545–1555 cm−1 is considered as a characteristic peak of the association between chitosan and metal [49,50]. Also, in the spectra of salt–polymer films, the appearance of an absorption band with a maximum at 827 cm−1 is characteristic. The appearance of this band is associated with the introduction of nickel nitrate and the formation of various nitro compounds [51].

Table 2.
Wavenumbers (cm−1) of main bands from FTIR spectra of the polymer (chitosan) and salt–polymer films.
Based on the analysis of the IR spectra of chitosan and precursors of composite materials, it can be assumed that a chelate complex compound is formed (Figure 3).
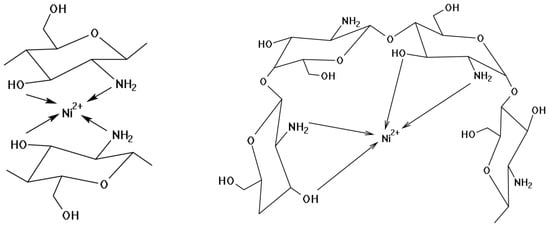
Figure 3.
Proposed structures of the fragment formed during the interaction between Ni2+ and chitosan.
Figure 4 shows the IR spectra of the composite materials after heat treatment in an Ar environment at 500 °C. It was found that heat treatment in an inert environment leads to the disappearance of the absorption bands in the region of OH and NH stretching vibrations (~3300 cm−1), as well as in the region of aliphatic structure stretching vibrations (~2900 cm−1). The absence of these absorption bands indicates profound destruction of chitosan, including decomposition of aliphatic structures. An intense broad absorption band is observed with a maximum at 1565 cm−1 and mutually overlapping bands in the region of 1100–1500 cm−1. The 1565 cm−1 band is probably due to stretching vibrations of C=C bonds in polyene chains, which are weakly active in the IR range [52]. And the overlapping bands in the region of 1100–1500 cm−1 are characteristic of various aromatic fragments [53].
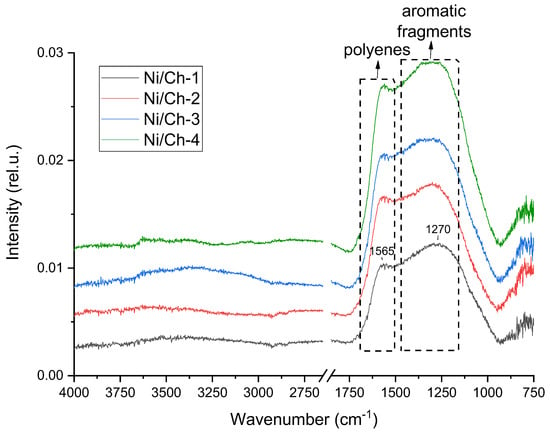
Figure 4.
IR Fourier spectroscopy of composite materials obtained by thermal decomposition of a mixture of nickel nitrate and chitosan.
2.1.5. Raman Spectroscopy
The composite materials were studied using Raman spectroscopy. The obtained spectra are shown in Figure 5. The Raman spectra contain two main typical bands characteristic of graphite-like materials. The G band has a maximum at ~1580 cm−1 and characterizes carbon in sp2-hybridization, characteristic of the C=C bond. The spectra also contain a band with a maximum at ~1374 cm−1, characteristic of the D band, caused by the presence of defects and corresponding to sp3-hybridization. Overlapping bands in the region of 3300–2300 cm−1 can be attributed to unresolved overtones of the D and G bands [54]. To analyze the structural defects, the intensity ratio of the D and G bands (ID/IG) was calculated, which is a key parameter for assessing the degree of defects. ID/IG for all studied samples varied within a narrow range from 0.85 to 0.95. The obtained data show that increasing the mass fraction of nickel in the composite does not lead to a significant change in the ID/IG value. This indicates that varying the Ni content within the studied limits does not have a significant effect on the defect density in the forming carbon matrix.
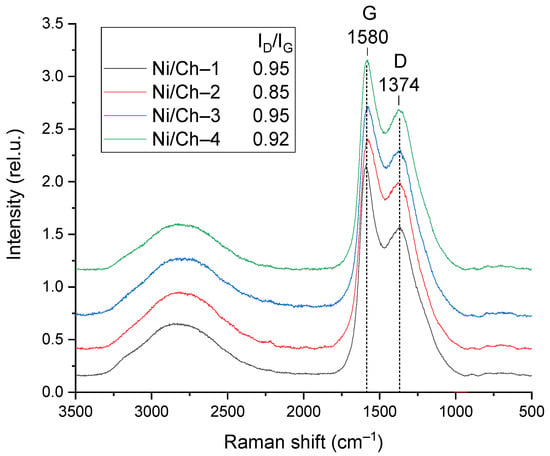
Figure 5.
Raman spectra of composite materials.
2.1.6. Morphology Characterization
The textural characteristics of the composite samples are presented in Table 3. For context, the specific surface area of the pure chitosan support subjected to the same thermal treatment is negligible (0.2 m2/g), highlighting the critical role of nickel in the pore-forming process. The obtained data demonstrate a clear dependence of the porosity parameters on the composition. The total specific surface area (Ssp) increases sharply from 26 m2/g for the Ni/Ch–1 sample to 257 m2/g for Ni/Ch–2. This indicates a sharp activation of the surface with an increase in the Ni(NO3)2 content in the composite precursors. A further increase in the Ni content also leads to changes. The surface reaches a maximum of 269 m2/g for Ni/Ch–3, and then decreases to 224 m2/g for the Ni/Ch–4 sample. A similar nonlinear dependence is observed for the contribution of micropores: their area (Smicro) and volume (Vmicro) are maximum for the Ni/Ch–2 sample (244 m2/g and 0.11 mL/g) and decrease noticeably with a further increase in the nickel content. Moreover, the parameters associated with mesopores (Smeso, Vmeso) and the outer surface (Sext) steadily increase with increasing Ni content. This leads to a consistent increase in the total pore volume (Vpore), but the average pore diameter (D) also varies nonlinearly, which is related to the distribution of micro- and mesopores.

Table 3.
Textural characteristics of composite materials.
The observed dynamics are likely related to the activation of the carbon component of the composites and are induced by Ni(NO3)2 [55]. During heat treatment of the salt–polymer films, the released gaseous nitrogen oxides act as an activating agent. The low Ni content (5 wt.% from chitosan) in the Ni/Ch–1 composite precursor proved insufficient for effective activation. A Ni concentration of 10 wt.% from chitosan (Ni/Ch–2) leads to intense but controlled gas evolution, forming a developed microporous surface with a minimal average pore size. Increasing the nickel content (Ni/Ch–3, Ni/Ch–4) causes more aggressive gas evolution, leading to partial destruction of the walls between micropores, their enlargement, and the formation of mesopores. This process explains the observed shift in pore distribution and a significant increase in average pore diameter. Thus, by varying the nickel content, it is possible to specifically control not only the specific surface area but also the pore size distribution in the composite material.
Figure 6 shows scanning microscopy images of Ni/Ch–2 and Ni/Ch–4 composite samples. Chitosan is known to have a thin lamellar structure with a smooth and nonporous surface [56,57]. SEM images confirmed the results presented in Table 3. The resulting composites have numerous cracks and a developed porous structure.
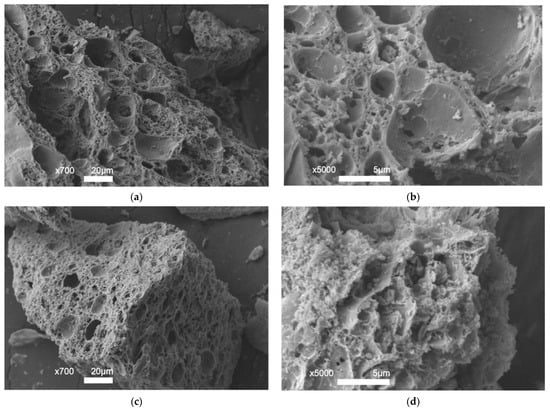
Figure 6.
SEM images of composite materials: (a,b) Ni/Ch–2; (c,d) Ni/Ch–4.
Figure 7 shows TEM images of the Ni/Ch–2 and Ni/Ch–4 composite samples. Detailed TEM examination of the samples reveals that the resulting composites consist of uniformly distributed Ni-containing particles in a carbon matrix. For the studied samples, the particle size does not exceed 12 nm. The Ni/Ch–2 sample is characterized by a more uniform particle size distribution, as the majority are in the 4–7 nm range, compared to 4–9 nm for Ni/Ch–4. The narrower particle size distribution for the Ni/Ch–2 sample is due to the less dense organization of the Ni-containing particles in the carbon matrix.
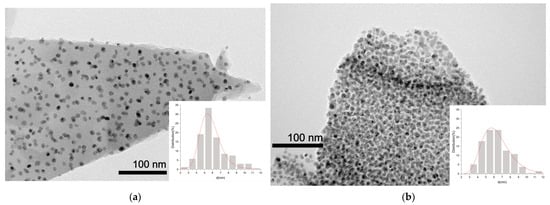
Figure 7.
TEM images of composite materials: (a) Ni/Ch–2; (b) Ni/Ch–4.
2.1.7. XPS
The qualitative and quantitative composition of the surface of the composite catalyst samples is presented in Table 4.

Table 4.
Qualitative and quantitative surface composition of composite materials.
Table 5 presents a summary of the XPS results of the surface composition of composite materials.

Table 5.
Summary of XPS results of the surface composition of composite materials.
The surface of the material consists of four elements: carbon, oxygen, nitrogen, and nickel. With an increase in the nickel content in the composite catalyst, a carbon matrix is formed, and for the Ni/Ch–1 (containing Ni 5 wt.% from chitosan), carbon on the surface is presented in the form of C-C (284.8 eV), C-O/sp2 C-N (285.6 eV), C=O/sp3 C-N (287.2 eV), and -COO-/CO32– (289.6 eV) groups [58]. The carbon matrix in the case of concentrations of 10–20 wt.% from chitosan is more graphitized—in the C 1 s spectra, there are bands from C=C (284.0–284.4 eV); it is also worth noting the π-π * shake-up (291.5–291.9 eV), which is characteristic of the formation of C=C conjugated structures [59,60]. It is worth noting separately that the surface of all composites is characterized by the presence of various oxygen-containing groups (C=O/COO-/CO32−/C-O (285.4–289.6 eV)), which indicates significant functionalization. The nitrogen content varies and depends on the nickel content in the composite. During the preparation of the material, nitrogen is incorporated into various carbon structures—pyrrole (399.6–400.3 eV), pyridine (397.9–398.5 eV), graphite (402.7 eV), and oxidized N (pyridine–N-O) (403.0–404.4 eV) [60,61]. The nickel structure on the surface undergoes the following metamorphoses: For the Ni/Ch–1 sample, nickel on the surface is an oxidized Ni2+ nanoparticle (854.1, 854.9 eV), which can also be associated with nitrogen NiNx/C [62], which can be partially hydrated (856.1 eV) [62]. Increasing the nickel content to 10–15 wt.% from chitosan promotes the formation of a metallic phase (852.5 eV), along with the oxide phase NiNx/C (854.8 eV) [63]. A further increase in the nickel content (up to 20 wt.% from chitosan) leads to the formation of a more complex surface structure: along with the metallic phase (851.8 eV), the formation of a transient oxidized phase Ni+ (852.4 eV) is recorded [64,65]. An oxide/hydroxide layer of Ni2+ was also recorded (854.9 eV). The oxygen content increased upon moving from low to higher nickel concentrations. Oxygen is predominantly represented in carbon-containing functional groups (532.8 eV–533.2 eV), hydroxide groups (531.2–531.4 eV), and metal–oxide bonds, which can be attributed to the boundary of the oxide particle (529.5–530.5 eV) [66,67]. The Ni/Ch–4 sample deserves special mention. In the oxygen spectrum, an oxygen signal related to the lattice oxygen of the oxide (528.9 eV) can also be distinguished [68].
Thus, it can be concluded that the resulting material may be a hybrid Ni-N-C material, which is a system with atomically dispersed NiNx active centers on an N-doped carbon matrix. By varying the nickel concentration, it is possible to actively influence the functional and chemical composition of the surface of the composite material.
2.2. Catalytic Activity
The synthesized composites are of practical interest as catalysts, which was demonstrated using the example of the thermal catalytic decomposition of methane. A key advantage of the developed materials is their ability to exhibit catalytic activity without a preliminary reduction step in a hydrogen stream, which is necessary for traditional oxide or supported Ni-containing catalysts [69,70,71]. This is made possible by the fact that the applied synthesis technique allows for the formation of the active phase of metallic nickel directly during the composite production stage. To evaluate the catalytic properties, the materials’ activity was screened in the methane decomposition reaction in the temperature range of 500–950 °C. The catalytic performance was assessed based on the methane conversion achieved after 15 min of reaction. Figure 8 shows the dependences of methane conversion on the experimental temperature for the studied samples.
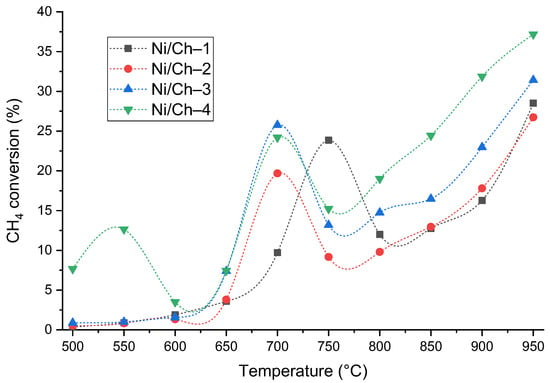
Figure 8.
Temperature dependence of methane conversion in the methane decomposition reaction for composite material samples.
An analysis of the temperature dependences of methane conversion (Figure 8) revealed general patterns and individual features of the catalytic behavior of the synthesized composites. All samples exhibited a nonmonotonic activity profile, typical of systems where activation and sintering processes compete with an increase in temperature. The initial increase in conversion (in the range of 500–650 °C) is likely associated with the activation and/or reduction of residual nickel oxide. A subsequent decrease in activity in the range of 750–800 °C is explained by the agglomeration of these particles, after which, at temperatures above 850 °C, a secondary increase in conversion is observed, probably due to the activity of the carbon product of methane decomposition in the same process. Moreover, the key differences correlate with the composition and texture of the materials. The Ni/Ch–1 sample, with the lowest Ni content, demonstrates the expected low activity in the low-temperature region. The Ni/Ch–2 and Ni/Ch–3 catalysts exhibit similar behavior, reaching maximum conversion (~24–26%) already at 700 °C, which may indirectly indicate the optimal number and dispersion of active sites. The most pronounced and complex dynamics are demonstrated by the Ni/Ch–4 sample with the highest nickel content. It is distinguished by significant initial activity at 500 °C and the presence of two conversion peaks (at 550 and 700 °C). This behavior may indicate the stepwise involvement of different forms of nickel in the process. Despite sintering at temperatures above 700 °C, this sample reaches methane conversion (37.2%) by 950 °C. However, conversion is not the only indicator of the methane decomposition process. The main process parameters at methane decomposition temperatures of 550 °C, 700 °C, and 950 °C are presented in Table 6.

Table 6.
Main indicators of catalytic decomposition of methane in the presence of composite catalysts based on chitosan.
In addition to methane conversion, two key parameters were calculated to evaluate the catalytic efficiency of the materials: activity (A), expressing the mass of H2 formed per unit mass of catalyst per hour, and hydrogen yield, characterizing the mass of H2 per unit mass of Ni per hour. At low temperature (550 °C), only the Ni/Ch–4 sample exhibits significant activity (0.32 gH2/gcat/h) and, most importantly, a high specific hydrogen yield at low temperature (0.68 gH2/gNi/h). This indirectly confirms the earlier conclusion that highly dispersed and easily activated nickel-based centers are formed in the materials already at the synthesis stage, which operate effectively even under mild conditions. For the remaining samples with a lower nickel content, the activity at this stage is negligible. In the region of the methane conversion peak (700 °C), all catalysts except Ni/Ch–1 demonstrate high activity (from 0.39 to 0.62 gH2/gcat/h). The maximum yield of H2 is observed for the Ni/Ch–2 and Ni/Ch–3 samples (1.90 and 1.98 gH2/gNi/h, respectively). This indicates that these composites are characterized not only by high dispersion of the active phase but also by the most efficient use of the introduced nickel. For Ni/Ch–4, despite the highest activity (0.62 gH2/gcat/h), the specific yield is significantly lower (1.30 gH2/gcat/h), which indicates a decrease in the efficiency of metal use with its excess. At high temperatures (950 °C), despite the increase in methane conversion and catalyst activity (A for Ni/Ch–4 reaches 0.95 gH2/gcat/h), the specific yield of hydrogen decreases with increasing nickel content in the composites. It can be assumed that under conditions of prolonged high-temperature exposure, carbon formed during the reaction begins to act as a catalyst [72,73]. Probably, the morphology and catalytic properties of the resulting carbon product depend on the amount of nickel in the composite. The composite with the lowest nickel content (Ni/Ch–1) demonstrates the highest specific yield under these conditions, which can be explained by the formation of an optimally structured carbon co-catalyst on its basis.
Thus, it can be concluded that the selection of the optimal composite material as a methane decomposition catalyst should be based on the process temperature used. For a low-temperature process (550 °C), the use of a composite with a high nickel content (Ni/Ch–4) is justified. For a medium-temperature process (700 °C), a material with an optimal metal balance (Ni/Ch–2 or Ni/Ch–3) is justified. And for a high-temperature process (950 °C), a system with a minimal amount of metal (Ni/Ch–1), where synergy with the forming carbon obtained from methane plays a key role.
Figure S2 (Supplementary Materials) shows the methane conversion dependence for the reduced composite catalyst samples. The Ni/Ch–3 and Ni/Ch–4 samples were chosen for the study because their TPR profiles exhibit the most pronounced hydrogen absorption peak in the 450–500 °C region, corresponding to the reduction of NiO to Ni. After preliminary reduction in a flow of H2, the low-temperature activity of the catalysts (up to 750 °C) decreases sharply. The conversion peaks observed for the initial composites in the 700–750 °C region completely disappear. Conversion begins to increase only above 800 °C, which is likely due to changes in the state and morphology of the active phase.
2.3. Characteristics of the Carbon Product
It is known that carbon of various structures can be formed during the catalytic decomposition of methane [74,75]. The Raman spectra of the obtained carbon product are shown in Figure 9. The spectra show two main peaks at about 1350 and 1580 cm−1, c corresponding to the D and G bands, respectively. There are also four less intense peaks in the region of 2400–3300 cm−1. The most pronounced second-order Raman peak is located at a frequency of ~2700 cm−1 and is called 2 D or G’ [76]. The obtained Raman spectra have the following distinctive features: the first-order bands are narrow, and there is also an intense second-order peak with a large number of shoulders. These distinctive features can be attributed to multi-walled carbon nanotubes (MWCNTs), which is consistent with the literature data, since in many studies, when using a nickel-containing catalyst, the carbon from methane decomposition is formed in the form of various carbon nanotubes [77,78,79].
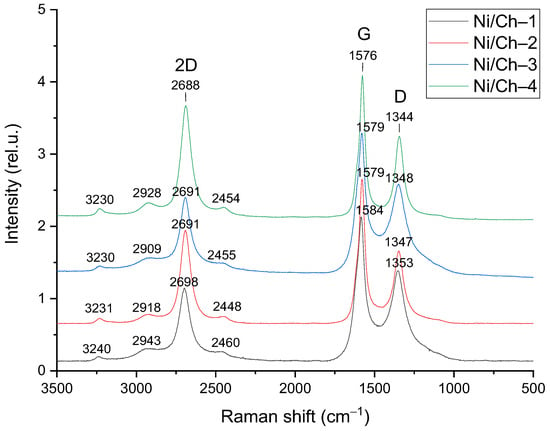
Figure 9.
Raman spectra of the carbon product obtained on composite catalysts.
Table 7 presents more detailed characteristics of the obtained Raman spectra. It was found that in a series of samples, an increase in the nickel content in the initial composites leads to a small but noticeable increase in the defectiveness of carbon nanotubes (an increase in the I2 D/IG ratio from 0.56 to 0.76). The presence of an intense second-order 2 D peak indicates the formation of MWCNTs [80]. The I2 D/IG ratio is a key parameter for indirectly assessing the number of layers in MWCNTs [81]. A higher I2 D/IG value usually corresponds to a greater number of ordered layers. In this case, the highest I2 D/IG value (1.34–1.43) was observed for carbon obtained on the composite with the highest Ni content (Ni/Ch–4). At the same time, the lowest I2 D/IG ratio (0.38–0.56) was obtained for the Ni/Ch–3 composite, which may indicate the formation of nanotubes with a smaller number of layers. Interestingly, no direct linear correlation is observed between the nickel content and the I2 D/IG ratio. This nonmonotonic behavior suggests that the number and perfection of carbon layers depend not only on the amount of active metal but also on other factors, such as the size of the nickel particles and the specific conditions of carbon deposition during growth.

Table 7.
Characteristics of Raman spectra of the carbon product of methane decomposition on composite catalysts.
3. Materials and Methods
3.1. Synthesis of Composite Materials
Composite catalysts were synthesized using matrix isolation. Chitosan was used as the carbon precursor, and nickel (II) nitrate hexahydrate (Analytical grade, Vekton, Moscow, Russia) was used for the nickel precursor.
In the first step of composite catalyst synthesis, a mixed solution of the polymer and the active metal salt was prepared. A 5 g sample of chitosan was dissolved in an aqueous acetic acid solution (0.125 M, 280 mL) with stirring and heating (45 °C), after which nickel (II) nitrate hexahydrate was added. The mixed solution was then dried to remove the solvent until a polymer film of constant weight was obtained. The nickel content in the polymer–salt mixture varied between 5 and 20% by weight of chitosan, calculated as pure nickel. The resulting film was then subjected to heat treatment in a tubular quartz furnace in an argon flow (10 L/h) for a total of 3 h: 60 °C—1 h (removal of nitric acid formed during the dissolution of nitrate salts), 100 °C—1 h (removal of residual water), and 500 °C—1 h (decomposition of nitrate and destruction of the polymer).
3.2. Sample Research Methods
3.2.1. Elemental Analysis
The Ni content in the samples was determined by flame atomic absorption analysis. A Perkin Elmer AAnalyst 400 atomic absorption spectrometer (Perkin Elmer, Waltham, MA, USA) with a relative error of 5–8% was used. Two types of flame were used: acetylene–air (~2300 °C) and nitrous oxide–acetylene (~3000 °C). Simultaneous determination of the C, H, and N content in the samples was carried out by chromatography after combustion of the sample in a dynamic Dumas flash. A Thermo Flash 2000 elemental analyzer (Thermo Fisher Scientific, Heysham, England, UK) was used.
3.2.2. Transmission and Scanning Electron Microscopy
The surface topography of the samples was assessed using a JEOL JIB-4501 scanning electron microscope (JEOL, Tokyo, Japan). A more detailed study of the sample morphology was performed using transmission electron microscopy (TEM) using a JEOL JEM 2100 electron microscope (JEOL, Tokyo, Japan).
3.2.3. Raman Spectroscopy
Raman spectra were recorded using a Bruker Senterra II confocal Raman spectrometer (Bruker, Billerica, MA, USA). A laser with a wavelength of 532 nm and a power of 0.25 mW was used. For the Raman spectra, ID/IG and I2 D/IG were calculated based on the deconvolution results in the Fityk program.
3.2.4. IR Spectroscopy
Measurement of optical reflection spectra in the IR range was carried out using a Fourier spectrometer IFS 66 v/s manufactured by Bruker (Bruker, Billerica, MA, USA).
3.2.5. XRD
X-ray diffraction analysis (XRD) was performed on a Tongda TD-3700 X-ray diffractometer (Dandong Tongda Science and Technology, Dandong, China), equipped with an X-ray tube with a copper anode, a Mythen2 R 1 K linear multichannel semiconductor detector, and a Goebel mirror, an X-ray optical element for forming a parallel beam. The average crystallite size was determined from the broadening of the observed diffraction maxima using the Debye–Scherrer equation.
3.2.6. The Surface Area
Specific surface area and pore size were determined using an ASAP 2020 V4.00 analyzer (Micromeritics, Norcross, GA, USA). Adsorption was carried out at −195.7 °C using N2 as the adsorbate. The pore size range was 0.35 to 500 nm. Specific surface area (SSA) was calculated using the Brunauer–Emmett–Teller (BET) method, and pore diameter and volume were calculated using the Barrett–Joyner–Halenda (BJH) method.
3.2.7. TPR-H2
Temperature programmed reduction (TPR–H2) of catalyst samples was carried out in a 5 mm diameter quartz flow reactor. Temperature was controlled using a chromel–alumel thermocouple. The temperature programming range was from room temperature to 700 °C, under atmospheric pressure. A flow of H2/Ar gas containing 5 vol.% hydrogen was supplied at a rate of 50 mL/min. The hydrogen content at the reactor outlet was determined using a thermal conductivity detector on a Crystallux–4000 M chromatograph (Meta–Chrom, Yoshkar-Ola, Russia). The sample weight for all catalyst samples was 30 mg.
3.2.8. XPS Spectroscopy
The study of the composite material samples’ surface was carried out by X-ray photoelectron spectroscopy on an X-ray photoelectron spectrometer (Prevac, Rogow, Poland). An X-ray tube with AlKα radiation (1486.6 eV) was used as a source of ionizing radiation. Before loading into the spectrometer, the samples were ground in an agate mortar and applied to conductive carbon tape. To neutralize the charge of the sample during the experiments, an electron–ion charge compensation system was used. All peaks were calibrated versus the C 1 s peak at 284.8 eV. The type of background was Shirley, and during deconvolution, it was assumed that the total peak was the sum of Gaussian curves.
3.3. Catalyst Activity Test
Catalytic tests of methane decomposition were conducted in a flow-through quartz reactor with a fixed catalyst bed. Methane (99.9 vol. %) was used as the feedstock. The reactor was set to a temperature of 500 °C (heating increments of 50 °C at 15 min intervals), after which methane was introduced (GHSV 1500 h−1). Each of the catalysts was tested without a preliminary activation step and under identical conditions.
The reactor outlet gas was analyzed online using a Kristallyuks-4000 M chromatograph (Meta-Chrom, Yoshkar-Ola, Russia). Separation of the gas mixture components was performed using a packed column with a Carboxen carbon adsorbent (column length 3 m; internal diameter 3 mm), with a thermal conductivity detector. The components were separated using a temperature-programmed mode (from 50 °C to 190 °C at a heating rate of 7.5 °C/min), using argon as the carrier gas (flow rate 30 mL/min). No gases other than hydrogen and methane were detected in the product gas.
The main process indicators were calculated using Equations (1)–(3).
4. Conclusions
Thus, in this study, new nickel/chitosan composite materials obtained using the organic matrix method were synthesized and characterized. A key feature of the proposed approach is the formation of an active nickel metallic phase directly during composite synthesis, eliminating the need for a mandatory catalyst pre-reduction step. Comprehensive characterization of the synthesized materials confirmed the chemical interaction between nickel ions and chitosan functional groups and also allowed us to establish a relationship between nickel content and textural characteristics. It was established that nitrate ion decomposition acts as an activating agent, enabling targeted modification of the specific surface area and micropore-to-mesopore ratio in the material.
A study of the catalytic properties in the thermocatalytic decomposition of methane revealed a dependence of activity on nickel content, which is determined by the target process temperature. The selection of the optimal Ni/Ch catalyst is determined by the target process temperature. The composite with the highest nickel content (Ni/Ch–4) demonstrates the highest efficiency at low temperatures (550 °C). In the mid-temperature range (700 °C), the most efficient metal utilization is observed for the Ni/Ch–2 and Ni/Ch–3 composites, while for high-temperature conditions (950 °C), the material with the lowest nickel content (Ni/Ch–1) proves most promising.
These results demonstrate the fundamental possibility of controlling catalytic properties through targeted design of the composition and texture of the composite material. The identified synergistic effect between the metal phase and the carbon matrix, as well as the formation of an active carbon co-catalyst during the reaction, opens up prospects for further research and the development of highly efficient catalysts for methane decomposition to produce hydrogen.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms27031255/s1.
Author Contributions
Conceptualization, M.K. and M.I.; methodology, M.I.; software, M.I. and A.S.; validation, M.K. and M.I.; formal analysis, A.S. and V.V.; investigation, A.S. and M.I.; resources, M.K.; data curation, A.S. and V.V.; writing—original draft preparation, A.S. and V.V.; writing—review and editing, M.K. and M.I.; visualization, A.S.; supervision, M.I.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the State Program of TIPS RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
This work was performed using the equipment of the Shared Research Center “Analytical center of deep oil processing and petrochemistry of TIPS RAS”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDM | Catalytic decomposition of methane |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| TPR–H2 | Temperature programmed reduction |
| SSA | Specific surface area |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| Ni/Ch | Composites synthesized by the matrix isolation method |
| GHSV | Gas hourly space velocity, h−1 |
| MWCNTs | Multi-walled carbon nanotubes |
| Ssp | Surface area, m2/g |
| Smeso | Mesopore surface area, m2/g |
| Smicro | Micropore surface area, m2/g |
| Sext | External surface area, m2/g |
| Vpore | Total pore volume, mL/g |
| Vmeso | Mesopore volume, mL/g |
| Vmicro | Micropore volume, mL/g |
| D | Pore size, nm |
| XCH4 | Methane conversion, % |
| A | Activity, gH2/gCat/h |
| Hydrogen content, vol. % | |
| Methane content, vol. % |
References
- Tsyganov, V.N. The Role of Innovative Materials and Nanotechnologies in the Development of Production Processes and Their Economic Impact. Environ. Manag. Issues 2024, 3, 7–15. [Google Scholar] [CrossRef]
- Elbakyan, L.; Zaporotskova, I. Composite Nanomaterials Based on Polymethylmethacrylate Doped with Carbon Nanotubes and Nanoparticles: A Review. Polymers 2024, 16, 1242. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Rodrigues, T.; das Neves, J.; Sarmento, B.; Langer, R.; Conde, J. Facts and Figures on Materials Science and Nanotechnology Progress and Investment. ACS Nano 2021, 15, 15940–15952. [Google Scholar] [CrossRef]
- Schaming, D.; Remita, H. Nanotechnology: From the Ancient Time to Nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology. Interface Sci. Technol. 2019, 28, 1–27. [Google Scholar] [CrossRef]
- Tretyakov, Y.D.; Goodilin, E.A. Key Trends in Basic and Application-Oriented Research on Nanomaterials. Russ. Chem. Rev. 2009, 78, 801–820. [Google Scholar] [CrossRef]
- Kulikova, M.V.; Zemtsov, L.M.; Sagitov, S.A.; Efimov, M.N.; Krylova, A.Y.; Karpacheva, G.P.; Khadzhiev, S.N. Fischer-Tropsch Synthesis in the Presence∣ of Cobalt-Containing Composite Materials Based on Carbon. Solid Fuel Chem. 2014, 48, 105–111. [Google Scholar] [CrossRef]
- Auyezkhanova, A.S.; Talgatov, E.T.; Akhmetova, S.N.; Jumekeyeva, A.I.; Naizabayev, A.A.; Zamanbekova, A.T.; Malgazhdarova, M.K. Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation. Molecules 2025, 30, 3820. [Google Scholar] [CrossRef]
- Gross, E.; Toste, F.D.; Somorjai, G.A. Polymer-Encapsulated Metallic Nanoparticles as a Bridge Between Homogeneous and Heterogeneous Catalysis. Catal. Lett. 2015, 145, 126–138. [Google Scholar] [CrossRef]
- Na, K.; Zhang, Q.; Somorjai, G.A. Colloidal Metal Nanocatalysts: Synthesis, Characterization, and Catalytic Applications. J. Clust. Sci. 2014, 25, 83–114. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A Review of the Interfacial Characteristics of Polymer Nanocomposites Containing Carbon Nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L.; Bandeira, C.F. Carbon Nanotube Buckypaper Reinforced Polymer Composites: A Review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Kulikova, M.V.; Ivantsov, M.I.; Sotnikova, A.E.; Samoilov, V.O. Catalytic Design of Matrix-Isolated Ni-Polymer Composites for Methane Catalytic Decomposition. Polymers 2023, 15, 2534. [Google Scholar] [CrossRef] [PubMed]
- Beyou, E.; Bourgeat-Lami, E. Organic–Inorganic Hybrid Functional Materials by Nitroxide-Mediated Polymerization. Prog. Polym. Sci. 2021, 121, 101434. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Nassar, E.J.; da Silva Barud, H.; Silva, J.M.; Rocha, L.A. Polymer and Biopolymer Organic-Inorganic Composites Containing Mixed Oxides for Application in Energy up- and down-Conversion. Opt. Mater. 2022, 134, 113189. [Google Scholar] [CrossRef]
- Bukharbayeva, F.; Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Akhmetova, S.; Jumekeyeva, A.; Naizabayev, A.; Kenzheyeva, A.; Danilov, D. The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation. Molecules 2024, 29, 4584. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.; Niehues, M.; Ravoo, B.J.; Studer, A. Synthesis and Immobilization of Metal Nanoparticles Using Photoactive Polymer-Decorated Zeolite L Crystals and Their Application in Catalysis. Adv. Synth. Catal. 2020, 362, 2245–2253. [Google Scholar] [CrossRef]
- Nagarjuna, R.; Sharma, S.; Rajesh, N.; Ganesan, R. Effective Adsorption of Precious Metal Palladium over Polyethyleneimine-Functionalized Alumina Nanopowder and Its Reusability as a Catalyst for Energy and Environmental Applications. ACS Omega 2017, 2, 4494–4504. [Google Scholar] [CrossRef]
- Madani, M.; Hosny, S.; Alshangiti, D.M.; Nady, N.; Alkhursani, S.A.; Alkhaldi, H.; Al-Gahtany, S.A.; Ghobashy, M.M.; Gaber, G.A. Green Synthesis of Nanoparticles for Varied Applications: Green Renewable Resources and Energy-Efficient Synthetic Routes. Nanotechnol. Rev. 2022, 11, 731–759. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-Based Nanocomposites and Their Applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef]
- Akhmetova, S.; Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Malgazhdarova, M.; Zhurinov, M.; Abilmagzhanov, A.; Jumekeyeva, A.; Kenzheyeva, A. How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications. Molecules 2024, 29, 3214. [Google Scholar] [CrossRef]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide Based Bionanocomposites, Properties and Applications: A Review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef]
- de Almeida, D.A.; Sabino, R.M.; Souza, P.R.; Bonafé, E.G.; Venter, S.A.S.; Popat, K.C.; Martins, A.F.; Monteiro, J.P. Pectin-Capped Gold Nanoparticles Synthesis In-Situ for Producing Durable, Cytocompatible, and Superabsorbent Hydrogel Composites with Chitosan. Int. J. Biol. Macromol. 2020, 147, 138–149. [Google Scholar] [CrossRef]
- Khazaei, A.; Khazaei, M.; Rahmati, S. A Green Method for the Synthesis of Gelatin/Pectin Stabilized Palladium Nano-Particles as Efficient Heterogeneous Catalyst for Solvent-Free Mizoroki–Heck Reaction. J. Mol. Catal. A Chem. 2015, 398, 241–247. [Google Scholar] [CrossRef]
- Biernat, M.; Woźniak, A.; Chraniuk, M.; Panasiuk, M.; Tymowicz-Grzyb, P.; Pagacz, J.; Antosik, A.; Ciołek, L.; Gromadzka, B.; Jaegermann, Z. Effect of Selected Crosslinking and Stabilization Methods on the Properties of Porous Chitosan Composites Dedicated for Medical Applications. Polymers 2023, 15, 2507. [Google Scholar] [CrossRef]
- Kishore, M.; Hussein, S.A.M.; Abdoon, F.M.; Bindu, G.N.H.; Hamad, A.A.; Saxena, K.K.; Raj R, G.; Kumar, A.; Srinivas, T. Chitosan–PVA Composite Reinforced with Cu-Decorated ZnO Nanoparticles for Photocatalytic Dye Degradation. Chem. Phys. Impact 2025, 11, 100953. [Google Scholar] [CrossRef]
- Kulikova, M.; Chudakova, M.; Ivantsov, M.; Kuz’min, A.; Krylova, A.; Maksimov, A. Properties of Cu-Co Composite Catalysts for Synthesis of Aliphatic Alcohols. J. Braz. Chem. Soc. 2021, 31, 287–298. [Google Scholar] [CrossRef]
- Zeng, H.; Ma, G.; Zheng, X.; Lin, D.; Zhang, J.; Li, D. Preparation, Application and Regeneration of Chitosan Composite Adsorbents for Arsenic Removal: A Review from a Sustainable Perspective. Int. J. Biol. Macromol. 2025, 330, 148156. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/Metal Nanocomposites for Biomedical Applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, A.; Khan, A.A.; Ahmad, E. Boosting Turquoise Hydrogen and Carbon Nanotube Production via Catalytic Methane Decomposition: Influence of Active Metal Ratios in Fe5–Co–Zn/γ-Al2O3 Nanocatalysts. Nanoscale Adv. 2025, 7, 5067–5079. [Google Scholar] [CrossRef]
- Horváth, A.; Németh, M.; Beck, A.; Olasz, D.; Serényi, M.; Sáfrán, G. Dramatic Interference of Au TEM Grid with Model and Supported MoNi/MgO Catalysts during High Temperature Reduction and Methane Decomposition. Mater. Today Nano 2025, 32, 100690. [Google Scholar] [CrossRef]
- Sotnikova, A.; Ivantsov, M.; Lubavina, V.; Kulikova, M. Production of Turquoise Hydrogen by Methane Decomposition in the Presence of Ni/Cu/C-Containing Composite Catalysts. Int. J. Hydrogen Energy 2025, 196, 152568. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Methane Decomposition over Ni, Co and Fe Based Monometallic Catalysts Supported on Sol Gel Derived SiO2 Microflakes. Chem. Eng. J. 2015, 262, 1009–1021. [Google Scholar] [CrossRef]
- Huang, J.; Liu, T.; Wang, K.; Huang, Z.; Wang, J.; Rokhum, S.L.; Li, H. Room-Temperature and Carbon-Negative Production of Biodiesel via Synergy of Geminal-Atom and Photothermal Catalysis. Environ. Chem. Lett. 2024, 22, 1607–1613. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, P.; Jiang, L.; Liu, S.; Yang, Y.; Li, Z.; Wu, P.; Zhang, Z.; Li, H. Electron Localization-Triggered Proton Pumping Toward Cu Single Atoms for Electrochemical CO2 Methanation of Unprecedented Selectivity. Adv. Mater. 2024, 36, e2311149. [Google Scholar] [CrossRef]
- Yang, M.; Gao, W.; Zhang, H.; Li, S.; Mikulčič, H.; Baeyens, J. Catalytic Methane Decomposition on an Fe-Catalyst, Coated on a Novel Fleece Reactor. Energy 2025, 335, 138340. [Google Scholar] [CrossRef]
- Kosari, M.; Portillo, S.B.; Xi, S.; Pedersen, A.; Talpade, A.; Li, F. Zr-Promoted Ni Nanoparticles in Mesoporous Silica Spheres (NiZr/MSiO2) for Catalytic Decomposition of Methane. Chem. Eng. J. 2025, 507, 160328. [Google Scholar] [CrossRef]
- Lubavina, V.V.; Sotnikova, A.E.; Krysanova, K.O.; Ivantsov, M.I.; Kulikova, M.V. Iron and Biochar-Based Catalysts (Fe/C) for Hydrogen Production by Methane Decomposition. Russ. J. Inorg. Chem. 2025, 70, 1493–1500. [Google Scholar] [CrossRef]
- Pati, S.; Jangam, A. Hydrogen Production from Catalytic Methane Decomposition Using Ni-Phyllosilicate Functionalized Zeolite Membrane Reactor. Surf. Interfaces 2025, 75, 107811. [Google Scholar] [CrossRef]
- Kim, N.Y.; Choi, D.S.; Kim, J.; Kim, D.W.; Joo, J.B. Synthesis of Waste-Derived Nickel-Supported Red Mud Catalysts Containing Various Ni–Fe Alloy Species for Fixed-Bed Catalytic Methane Decomposition. J. Environ. Chem. Eng. 2025, 13, 118461. [Google Scholar] [CrossRef]
- Gazi, M.J.; Bahuguna, A.; Panda, S.; Rana, B.S.; Naik, D.V.; Bhandari, S.; Bordoloi, A. Synthesis of Ni-Fe Particle Embedded CNx Matrix for Efficient Catalytic Methane Decomposition. Int. J. Hydrogen Energy 2025, 101, 1518–1531. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A Review of Methane Pyrolysis Technologies for Hydrogen Production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G.; Hu, H.; Ma, X. Hydrogen Production by Catalytic Methane Decomposition: Carbon Materials as Catalysts or Catalyst Supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Tian, M.; Li, K.; Zhu, X.; Wei, Y.; Zheng, Y.; Zhang, L.; Long, Y.; Wang, H. Modified Al@Al2O3 Phase Change Materials by Carbon via In-Situ Catalytic Decomposition of Methane. Sol. Energy Mater. Sol. Cells 2019, 200, 109924. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR Studies of Chitosan Blends. Thermochim. Acta 2003, 396, 153–166, Erratrum in Thermochim. Acta 2004, 409, 95–97. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–Starch Composite Film: Preparation and Characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-Metal Complexes as Antimicrobial Agent: Synthesis, Characterization and Structure-Activity Study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Tang, L.-G.; Hon, D.N.-S. Chelation of Chitosan Derivatives with Zinc Ions. II. Association Complexes of Zn2+ OntoO,N-Carboxymethyl Chitosan. J. Appl. Polym. Sci. 2001, 79, 1476–1485. [Google Scholar] [CrossRef]
- Tarasevich, B.N. IR Spectra of the Main Classes of Organic Compounds; MSU: Moscow, Russia, 2012. [Google Scholar]
- Kaczmarek, H.; Zawadzki, J. Chitosan Pyrolysis and Adsorption Properties of Chitosan and Its Carbonizate. Carbohydr. Res. 2010, 345, 941–947. [Google Scholar] [CrossRef]
- Zheng, A.; Jiang, L.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; Wang, X.; He, F.; Li, H. Impact of Torrefaction on the Chemical Structure and Catalytic Fast Pyrolysis Behavior of Hemicellulose, Lignin, and Cellulose. Energy Fuels 2015, 29, 8027–8034. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Lieder, M.; Jablonska, M. N-Doped Mesoporous Carbon Nanosheets Obtained by Pyrolysis of a Chitosan–Melamine Mixture for the Oxygen Reduction Reaction in Alkaline Media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Hu, J.; He, Y.; Zhang, J.; Chen, L.; Zhou, Y.; Zhang, J.; Tao, H.; Zhou, N.; Mi, B.; Wu, F. In-Situ Catalytic Pyrolysis of Biomass with Nickel Salts: Effect of Nickel Salt Type. J. Anal. Appl. Pyrolysis 2023, 172, 106029. [Google Scholar] [CrossRef]
- Trimukhe, K.; Varma, A. A Morphological Study of Heavy Metal Complexes of Chitosan and Crosslinked Chitosans by SEM and WAXRD. Carbohydr. Polym. 2008, 71, 698–702. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of Chitosan Composites with Various Clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Dementjev, A.; de Graaf, A.; van de Sanden, M.C.; Maslakov, K.; Naumkin, A.; Serov, A. X-Ray Photoelectron Spectroscopy Reference Data for Identification of the C3 N4 Phase in Carbon–Nitrogen Films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Kvon, R.I.; Il’inich, G.N.; Chuvilin, A.L.; Likholobov, V.A. XPS and TEM Study of New Carbon Material: N-Containing Catalytic Filamentous Carbon. J. Mol. Catal. A Chem. 2000, 158, 413–416. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.R.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS Spectra for Nitrogen-Doped Chars: An Analysis from First Principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Tian, K.; Wang, J.; Cao, L.; Yang, W.; Guo, W.; Liu, S.; Li, W.; Wang, F.; Li, X.; Xu, Z.; et al. Single-Site Pyrrolic-Nitrogen-Doped Sp2-Hybridized Carbon Materials and Their Pseudocapacitance. Nat. Commun. 2020, 11, 3884. [Google Scholar] [CrossRef]
- Zhang, C.; Shahcheraghi, L.; Ismail, F.; Eraky, H.; Yuan, H.; Hitchcock, A.P.; Higgins, D. Chemical Structure and Distribution in Nickel–Nitrogen–Carbon Catalysts for CO2 Electroreduction Identified by Scanning Transmission X-Ray Microscopy. ACS Catal. 2022, 12, 8746–8760. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New Interpretations of XPS Spectra of Nickel Metal and Oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Barroso, N.; Galetti, A.E.; Arrúa, L.A.; Cornaglia, L.; Abello, M.C. XPS Study of the Surface Properties and Ni Particle Size Determination of Ni-Supported Catalysts. Surf. Interface Anal. 2014, 46, 521–529. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, S.; Li, Y.; Jia, C.; Su, Z.; Hao, D.; Ni, B.; Zhang, Q.; Zhao, C. Heterostructured V-Doped Ni2P/Ni12P5 Electrocatalysts for Hydrogen Evolution in Anion Exchange Membrane Water Electrolyzers. Small 2022, 18, e2204758. [Google Scholar] [CrossRef]
- Easton, C.D.; Morgan, D.J. Critical Examination of the Use of X-Ray Photoelectron Spectroscopy (XPS) O 1 s to Characterize Oxygen Vacancies in Catalytic Materials and Beyond. J. Vac. Sci. Technol. A 2025, 43, 053205. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, Y.; Lai, X.; Guo, Q.; Zhao, J.; Yang, Y.; Li, Y. Hierarchical Structure N, O-Co-Doped Porous Carbon/Carbon Nanotube Composite Derived from Coal for Supercapacitors and CO2 Capture. Nanoscale Adv. 2020, 2, 878–887. [Google Scholar] [CrossRef]
- Blume, A.R.; Calvet, W.; Ghafari, A.; Mayer, T.; Knop-Gericke, A.; Schlögl, R. Structural and Chemical Properties of NiOx Thin Films: The Role of Oxygen Vacancies in NiOOH Formation in a H2O Atmosphere. Phys. Chem. Chem. Phys. 2023, 25, 25552–25565. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Kim, K.C.; Kim, M.-J.; Youn, J.-R.; Kim, M.; Jung, T.; Jeon, S.G.; Kim, W.; Ko, C.H. Production of CO2-Free Hydrogen via Catalytic Methane Decomposition over Ce-Promoted Ni/Al2O3 Catalysts. Fuel Process. Technol. 2025, 278, 108338. [Google Scholar] [CrossRef]
- Bibak, F.; Meshkani, F. Promotion Effect of the Rare Earth Metals (Cerium, Lanthanum, and Yttrium) on the Catalytic Performance of Ni/Al2O3 Catalyst Fabricate via the Neoteric One-Pot Hydrothermal Method for Methane Decomposition. Fuel 2024, 366, 131048. [Google Scholar] [CrossRef]
- Tenório, N.V.N.; Motta, R.J.B.; Meneghetti, S.M.P.; de Almeida, R.M. Application of the Modified Pechini Method Through the Addition of Glycerol in the Synthesis of Fe/Al2O3 and Ni/Al2O3 Catalysts for Methane Decomposition. Waste Biomass Valorization 2025. [Google Scholar] [CrossRef]
- Gubanov, M.A.; Ivantsov, M.I.; Kulikova, M.V.; Kryuchkov, V.A.; Nikitchenko, N.V.; Knyazeva, M.I.; Kulikov, A.B.; Pimenov, A.A.; Maksimov, A.L. Methane Decomposition Nickel Catalysts Based on Structured Supports. Pet. Chem. 2020, 60, 1043–1051. [Google Scholar] [CrossRef]
- Sotnikova, A.; Ivantsov, M.; Kulikov, A.; Kulikova, M. Influence of MgO Promoution to Ni-Based Composites in Hydrogen Production Methane Decomposition Process. Int. J. Hydrogen Energy 2024, 57, 1208–1220. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- MURADOV, N.; VEZIROLU, T. From Hydrocarbon to Hydrogen?Carbon to Hydrogen Economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- López-Díaz, D.; Delgado-Notario, J.A.; Clericò, V.; Diez, E.; Merchán, M.D.; Velázquez, M.M. Towards Understanding the Raman Spectrum of Graphene Oxide: The Effect of the Chemical Composition. Coatings 2020, 10, 524. [Google Scholar] [CrossRef]
- Kazemi, S.; Alavi, S.M.; Rezaei, M. Influence of Various Spinel Materials Supported Ni Catalysts on Thermocatalytic Decomposition of Methane for the Production of COx-Free Hydrogen. Fuel Process. Technol. 2023, 240, 107575. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Deyab, M.A.; Azab, M.A.; Haggar, A.M. Impact of Cr Doping on the Performance of Ni/Al2O3 Catalyst through Methane Decomposition into COx-Free Hydrogen and Carbon Nanomaterials. Chem. Eng. Res. Des. 2022, 186, 701–712. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Alotaibi, M.A.; Abdel-Fattah, E.M.; Qahtan, T.F.; Alshreef, O.A. The Role of Promoters on NiO Catalysts for Methane Decomposition and Hydrogen Production. Sci. Rep. 2025, 15, 21448. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, J.; Wang, Y.; Dang, Y.; Heumann, S.; Ding, Y. Insights into the Role of Defects on the Raman Spectroscopy of Carbon Nanotube and Biomass-Derived Carbon. Carbon 2024, 222, 118998. [Google Scholar] [CrossRef]
- Singh, K.; Chaudhary, S.; Venugopal, R.; Gaurav, A. Bulk Synthesis of Multi-Walled Carbon Nanotubes by AC Arc Discharge Method. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2017, 231, 141–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.