Abstract
The quality of high-density lipoproteins (HDLs) is characterized by lipid and protein composition, oxidation and glycation extent, and particle size, while the quantity of HDL-C is just the cholesterol amount in HDL. The inverse association between HDL-C and cardiovascular disease (CVD) and hypertension has been well established; however, the U-shaped mortality risk observed from HDL-C underscores that HDL quality and function are equally important. The present cross-sectional study assessed the correlations of serum lipid and glucose profiles, and low-density lipoprotein (LDL) and HDL characteristics, with blood pressure (BP) distribution in ordinary middle-aged Korean participants (n = 50; mean age 47.0 ± 11.7 years; males: n = 25, 49.2.0 ± 11.7 years; females: n = 25, 44.8 ± 11.5 years), with particular focus on HDL quality and its antioxidant capacity. This study observed that serum elevated triglyceride (TG) and glucose levels were directly proportional to elevated systolic BP (SBP) and diastolic BP (DBP), whereas serum total cholesterol (TC), LDL-C, and HDL-C were not correlated with BP. However, HDL-C/TC (%) was negatively associated with SBP (p = 0.036), while TG/HDL-C and glucose/HDL-C ratios were positively associated with both SBP and DBP, suggesting that TG and glucose proportions relative to HDL-C are probable predictors of hypertension. Elevations of TG, oxidation, and glycation in LDL were positively associated with elevations of BP, whereas LDL particle size was negatively correlated with BP. Similarly, elevations of TG and glycation in HDL2 and HDL3 were positively correlated with elevations of BP, while the particle size of HDL2 was negatively correlated with BP. The heightened HDL2-associated paraoxonase (PON) activity and ferric ion reduction ability (FRA) negatively correlated with LDL oxidation and particle size, whereas elevated HDL3-associated PON and FRA activities were inversely related to LDL glycation. An enhanced glycation in HDL2 was negatively correlated with HDL2-associated PON activity and FRA, while an increase in HDL2 particle size was only dependent on the associated PON activity but not on FRA. In conclusion, observational outcomes demonstrated that improved HDL quality and functionality (characterized by large particle size, reduced glycation, and higher FRA and PON activities) were inversely correlated with LDL oxidation, glycation, particle shrinkage, and the risk of hypertension.
Keywords:
high-density lipoproteins; HDL; glucose; glycation; oxidation; paraoxonase; PON; Ferric ion reduction ability; FRA; hypertension 1. Introduction
It is well known that serum high-density lipoprotein cholesterol (HDL-C) levels are inversely associated with the risk of cardiovascular disease, hypertension, stroke, and diabetes mellitus [1,2,3]. Also, higher HDL-C levels are linked to a lower incidence of metabolic syndrome [4]. This inverse relationship between blood pressure (BP) and HDL-C has been observed in many national health and nutrition examination surveys, in which individuals with higher HDL-C have lower systolic BP (SBP) and diastolic BP (DBP) [5,6]. Nevertheless, it has also been reported that extremely high levels of HDL-C are not always beneficial and can be associated with an increased risk of cardiovascular disease (CVD) and mortality [7,8]. A U-shaped relationship between HDL-C and all-cause mortality suggests that extremely low and high HDL-C levels are associated with increased mortality, while moderate to high levels of 73 mg/dL and 93 mg/dL for men and women, respectively, are associated with the lowest all-cause mortality [9]. These studies suggest that HDL quality, rather than HDL-C quantity, may play a more important role in determining its beneficial functions. This notion is supported by evidence showing that excessively high HDL-C levels are associated with increased all-cause mortality [10,11]. Moreover, studies have shown that poor-quality or dysfunctional HDL can exacerbate pro-atherogenic processes, thereby increasing the risk of cardiovascular mortality and hypertension, particularly in men [12,13].
High-quality HDL is characterized by higher cholesterol and apolipoprotein (apo)A-I content, spherical morphology, larger particle size, and resistance to glycation and oxidative damage [14]. Its functionality is mainly reflected in strong antioxidant capacity, such as paraoxonase (PON) activity and ferric ion reduction ability (FRA), and effective cholesterol efflux from atherosclerotic plaque [7]. In contrast, dysfunctional HDL is triglyceride (TG)-enriched, smaller, morphologically distorted, and more susceptible to glycation and oxidation [15], with reduced apoA-I content, weakened FRA and PON activities, and impaired cholesterol efflux capacity [16]. Consequently, dysfunctional HDL fails to protect LDL from oxidation and glycation, and it increases the formation of small dense LDL (sdLDL) [17]. These sdLDL particles are highly atherogenic due to their enhanced susceptibility to oxidative and glycation stress [18], enriched in fatty acids in TG, and depleted in antioxidant activity [19,20], with apolipoprotein (apo) B-100 fragmentation and reduced particle size [21]. Both sdLDL and glycoxidized LDL are independent risk factors for CVD and metabolic disease. Although HDL is known to protect LDL, the specific roles of HDL2 and HDL3 in preventing LDL from glycation and oxidation insult have not been fully elucidated.
Prior studies have focused primarily on the impact of HDL quality on atherosclerotic outcomes, and not much on the hypertension risk. Furthermore, population-specific evidence is critical in cardiometabolic research, as HDL function and lipoprotein profiles vary across ethnicities and metabolic backgrounds [22,23]. Specifically, studies with Korean participants with HDL functionality and hypertension are not extensively conducted. In this regard, the present study attempts to explore the HDL functionality–sdLDL–hypertension axis in the middle-aged Korean population.
In the current study, we examined the correlations of serum lipids, glucose profile, and lipoprotein (LDL, HDL2, and HDL3) properties with SBP and DBP in middle-aged Korean participants. Moreover, this study investigates the influence of HDL quality and functionality parameters, including particle size, cholesterol and TG content, glycation extent, and antioxidant abilities, on the incidence of pre-hypertension.
2. Results
2.1. Distribution of Age, Blood Pressure, and Body Mass Index
All participants showed normal distribution with a middle-aged population around 47.0 ± 11.7 years without differences between genders (Table 1). The combined BP of the participants showed a pre-hypertension stage of SBP around 126 ± 15.8 mmHg; however, the outcomes of the female group showed a normotensive range of SBP and DBP. The male group showed 11% and 14% higher SBP and DBP, respectively, than those of the female group. Anthropometric analysis revealed that the male group showed an obese range (BMI ≥ 25), with a 1.2-fold higher body mass index (BMI) than the female group, which was in the normal range, although the cumulative BMI from the total participants showed an overweight range based on the classification of the Asian-Specific BMI guidelines (>BMI 23.0–24.9). The female group showed 12% higher body fat (%) than the male group (p = 0.019); however, the male group showed 61% higher visceral fat than the female group (p < 0.001).

Table 1.
Anthropometric data, serum lipids, and lipoprotein profile of participants.
2.2. Correlation of Blood Lipid Profiles and Blood Pressure
As shown in Figure 1A,B, an increase in serum TG was positively correlated with an increase in both SBP (r = 0.575, p < 0.001) and DBP (r = 0.553, p < 0.001), although an increase in serum TC was not correlated with an increase in SBP (r = 0.222, p = 0.121) and DBP (r = 0.117, p = 0.419) (Table 2, Supplementary Figure S1A,B). Similar to TC, a non-significant correlation of LDL-C was noticed with SBP and DBP (Supplementary Figure S1C,D). A negative non-significant correlation between serum HDL-C levels and SBP (r = −0.238, p = 0.096) and DBP (r = −0.234, p = 0.102) was observed (Supplementary Figure S1E,F). The serum TG/HDL-C ratio was significantly and positively correlated with SBP (r = 0.476, p < 0.001) and DBP (r = 0.418, p = 0.002) as shown in Figure 1C,D. The HDL-C/TC (%) was negatively correlated with only SBP with significance (r = −0.298, p = 0.036), although HDL-C/TC (%) showed a non-significant correlation (r = −0.243, p = 0.089) with DBP. These results revealed that BP was more closely associated with serum TG (mg/dL), TG/HDL-C (ratio), and HDL-C/TC (%) rather than the individual levels of serum TC (mg/dL) or HDL-C (mg/dL). The outcomes suggesting a mutual ratio of TC, TG, and HDL-C are more important than the absolute and independent quantity of TC, TG, and HDL-C.
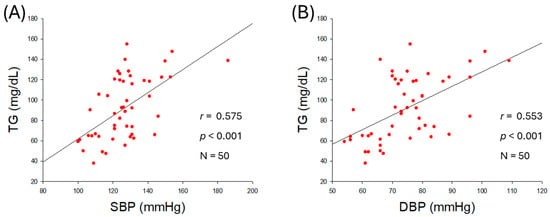
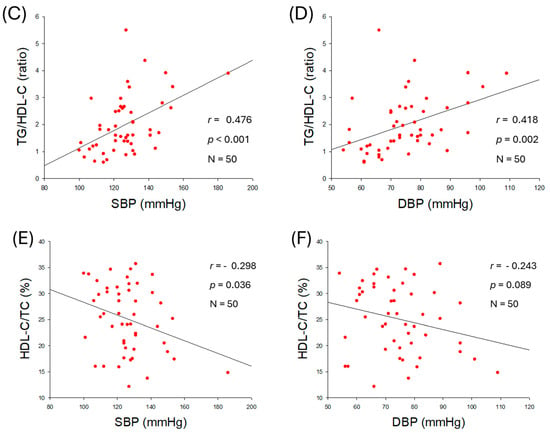
Figure 1.
Correlation analysis of serum lipid profile and blood pressure (BP). (A) Regression analysis between serum triglyceride (TG) and systolic blood pressure (SBP). (B) Regression analysis between serum TG and diastolic blood pressure (DBP). (C) Regression analysis between serum TG/high-density lipoprotein cholesterol (HDL-C) and SBP. (D) Regression analysis between serum TG/HDL-C and DBP. (E) Regression analysis between serum HDL-C/total cholesterol (TC) (%) and SBP. (F) Regression analysis between serum HDL-C/TC (%) and DBP.

Table 2.
Pearson correlation analysis of anthropometric, serum lipid, and lipoprotein parameters with SBP and DBP.
2.3. Correlation of Serum Glucose and Blood Pressure
As shown in Figure 2A,B, serum glucose level was positively associated with an increase in SBP (r = 0.525, p < 0.001) and DBP (r = 0.504, p < 0.001), suggesting that hyperglycemia is associated with hypertension. The ratio of glucose/HDL-C was also positively correlated with SBP (r = 0.399, p = 0.004) and DBP (r = 0.352, p = 0.012) as shown in Figure 2C,D, indicating that both hyperglycemia and reduced HDL-C level with respect to blood glucose are intimately associated with the severity of hypertension.
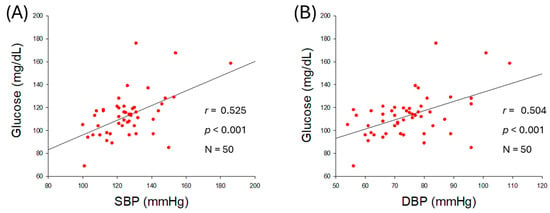
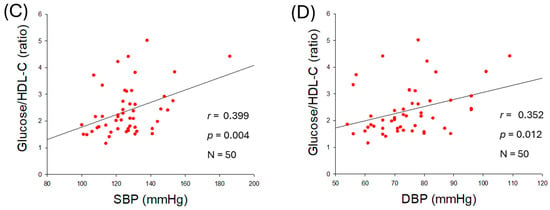
Figure 2.
Correlation analysis of serum glucose level and blood pressure (BP). (A) Regression analysis between serum glucose level and systolic blood pressure (SBP). (B) Regression analysis between serum glucose level and diastolic blood pressure (DBP). (C) Regression analysis between serum glucose/high-density lipoprotein cholesterol (HDL-C) ratio and SBP. (D) Regression analysis between serum glucose/HDL-C ratio and DBP.
2.4. Correlation of LDL Quality and Blood Pressure
As shown in Figure 3A,B, an increase in TG content in LDL was positively associated with SBP (r = 0.506, p < 0.001) and DBP (r = 0.448, p = 0.002), suggesting that an increase in TG in LDL, which reflects impairment of LDL quality, was correlated with hypertension. Moreover, as shown in Figure 3C,D, the oxidized extent of LDL was more positively associated with an increase in SBP (r = 0.617, p < 0.001) and DBP (r = 0.672, p < 0.001). These results suggest that high prevalence of oxidized LDL and TG-enriched LDL is associated with exacerbation of hypertension.
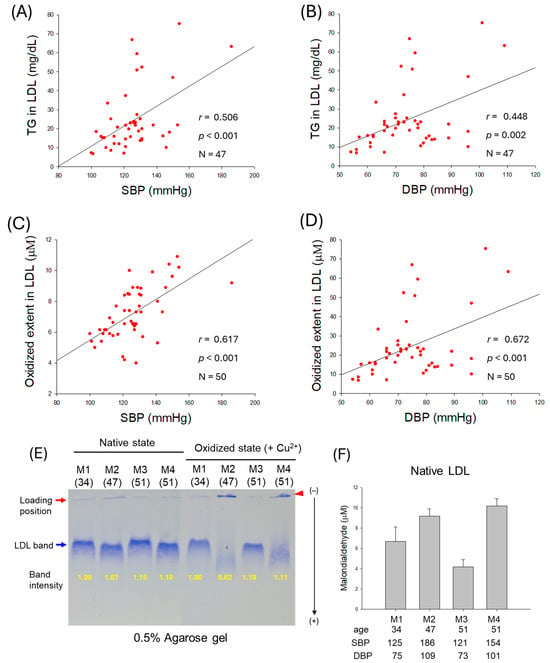
Figure 3.
Correlation of low-density lipoprotein (LDL) quality and blood pressure (BP). (A,B) Regression analysis of triglyceride (TG) content in LDL with systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively; the data for TG is from only 47 participants, as data from 3 participants showed some inconsistency due to lower volume of LDL. (C) Regression analysis of oxidized extent in LDL and SBP. (D) Regression analysis of oxidized extent in LDL and DBP. (E) Representative electrophoretic patterns of LDL from typical male normotensive participants (M1 and M3, 34- and 51-year-old, respectively) and hypertensive participants (M2 and M4, 47- and 51-year-old, respectively) under native state and oxidized state (in the presence of Cu2+). (F) Quantification of oxidized species in native LDL using thiobarbituric acid reactive substance (TBARS) assay with malondialdehyde (MDA) standard.
As depicted in Figure 3E, electrophoretic analysis of LDL from typical male normotensive participants (M1 and M3) and hypertensive participants (M2 and M4) was performed. The results revealed that hypertensive participants showed weaker band intensity and faster electromobility than normotensive participants in both native and oxidized states (induced by CuSO4). Especially in the oxidized state, under CuSO4 treatment (final 10 μM), LDL from hypertensive participants almost disappeared, as indicated by the blue arrow, due to the oxidation, while aggregated bands were detected in loading position, as indicated by the red arrowhead. Furthermore, the quantification of oxidized species in LDL using a malondialdehyde (MDA) standard by thiobarbituric acid reactive substances (TBARSs) revealed that hypertensive partcipants showed substantially higher MDA levels than normotensive participants (Figure 3F).
2.5. Particle Size and Glycation Extent of LDL
As shown in Figure 4A,B, particle size of LDL was negatively correlated with SBP (r = −0.436, p = 0.002) and DBP (r = −0.501, p < 0.001), suggesting that smaller LDL particle size is more associated with risk of hypertension. Furthermore, as shown in Figure 4C,D, glycation extent in LDL was positively associated with an increase in SBP (r = 0.464, p < 0.001) and DBP (r = 0.542, p < 0.001), suggesting that higher glycation in LDL is associated with risk of hypertension, especially DBP. These results indicate that a smaller particle size of LDL with higher glycation and oxidation is correlated with an increase in hypertension risk.
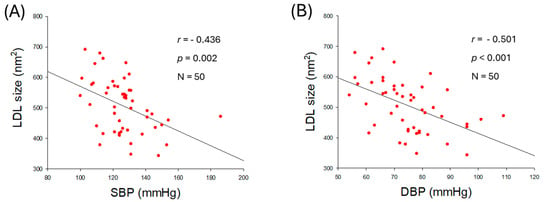
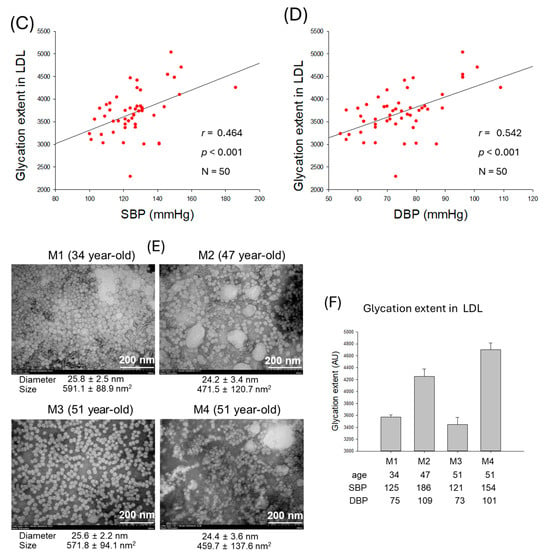
Figure 4.
Correlation analysis of qualities of low-density lipoprotein (LDL) particles and blood pressure (BP). (A) Regression analysis of LDL particle size and systolic blood pressure (SBP). (B) Regression analysis of LDL particle size and diastolic blood pressure (DBP). (C) Regression analysis of glycation extent of LDL and SBP. (D) Regression analysis of glycation extent of LDL and DBP. (E) Transmission electron microscope (TEM) image of representative male participants. (F) Glycation extent of LDL from selected male participants. AU, arbitrary unit.
The observation of LDL particles using transmission electron microscopy (TEM) revealed that normotensive participants (M1 and M3) showed 1.3-fold larger LDL particles, around 571~591 nm2 particle size, than hypertensive participants (M2 and M4), around 459~471 nm2 (Figure 4E, Supplementary Figure S2A–D). Furthermore, LDL images from the hypertensive participants (M2 and M4) exhibited smaller particle numbers, aggregation, and ambiguous morphology with distorted particle shape, whereas LDL images from the normotensive participants (M1 and M3) displayed larger particle numbers that are well separated, with clear spherical morphology. The extent of glycation revealed that normotensive participants (M1 and M3) harbor significantly lower glycation than hypertensive participants (M2 and M4) (Figure 4F). These results suggest the poor quality of LDL, marked by smaller particle size, distorted morphology, a higher degree of oxidation, and glycation directly linked to hypertension.
2.6. HDL2 Quality and Correlation with Blood Pressure
As shown in Figure 5A,B, an increase in TG content in HDL2 was positively associated with the elevation of SBP (r = 0.474, p < 0.001) and DBP (r = 0.444, p = 0.002), while TC content in HDL2 was not significantly associated with the elevation of SBP and DBP, although they showed notable negative regression (Supplementary Figure S3A,B). Increased glycation in HDL2 was positively associated with the elevation of SBP (r = 0.557, p < 0.001) and DBP (r = 0.557, p < 0.001) with remarkable significance (Figure 5C,D), suggesting that higher glycation in HDL2 is a risk factor of hypertension.
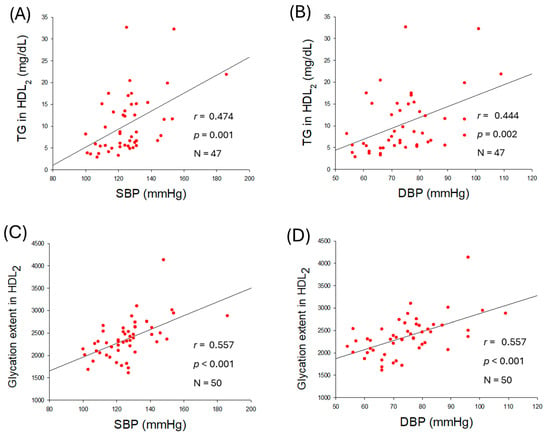
Figure 5.
Correlation analysis of high-density lipoprotein (HDL2) quality and blood pressure (BP). (A,B) Regression analysis of triglyceride (TG) content in HDL2 with systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively; the data for TG is from only 47 participants, as data from 3 participants showed some inconsistency due to lower volume of HDL2. (C) Regression analysis of glycation extent in HDL2 and SBP. (D) Regression analysis of glycation extent in HDL2 and DBP.
2.7. Correlation of HDL2 Size and Blood Pressure
As shown in Figure 6A,B, particle size of HDL2 was negatively correlated with SBP (r = −0.486, p < 0.001) and DBP (r = 0.−443, p = 0.001), suggesting that smaller particle size of HDL2 is more associated with risk of hypertension. The TEM image revealed that normotensive participants (M1 and M3) showed a bigger size, around 123–126 nm2, and distinct particle morphology with higher particle numbers. In contrast, hypertensive participants (M2 and M4) showed a smaller size, around 111–118 nm2, with aggregated and ambiguous particle morphology (Figure 6C, Supplementary Figure S4A–D). These results indicate that particle size and morphology of HDL2 are correlated with the incidence of hypertension: bigger particle size, more particle numbers, and a clean shape are desirable morphologies for healthy SBP and DBP. Furthermore, electrophoresis (15% SDS-PAGE) of HDL2 revealed a remarkably decreased apoA-I band in hypertensive participants (M2 and M4) that was quantified to be around 39~54% less than the apoA-I intensity quantified in the normotensive participants (M1 and M3) (Figure 6D).
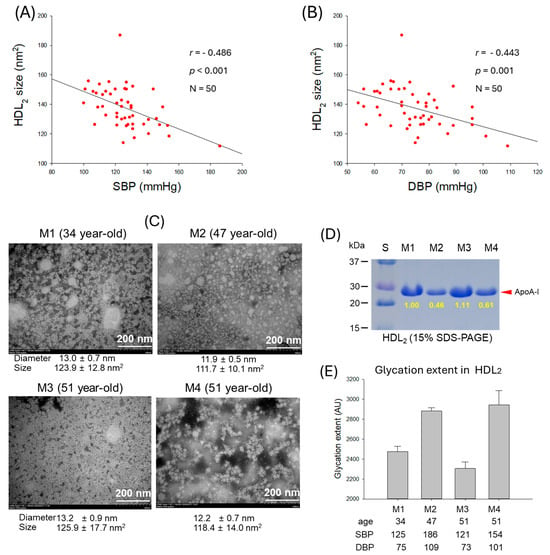
Figure 6.
Correlation analysis of high-density lipoprotein (HDL2) particle properties and blood pressure (BP). (A) Regression analysis of HDL2 particle size and systolic blood pressure (SBP). (B) Regression analysis of HDL2 particle size and diastolic blood pressure (DBP). (C) Transmission electron microscope (TEM) images of the representative male participants, comparing between normotensive (M1 and M3) and hypertensive (M2 and M4). (D) Electrophoretic profile of HDL2 to compare band intensity of apolipoprotein (apo)A-I (15% SDS-PAGE). (E) Glycation extent of HDL2 from the representative male participants. AU, arbitrary unit.
A lower degree of HDL2 glycation was observed in the normotensive participants (M1 and M3), whereas the hypertensive participants (M2 and M4) showed markedly higher glycation levels (Figure 6E). These findings suggest that HDL2 glycation is correlated with both SBP and DBP. As depicted in Figure 6E, M2 exhibited a 1.2-fold increase in HDL2 glycation compared with M1, which corresponds to the 1.5-fold higher SBP and DBP in these participants. Similarly, M4 displayed a 1.2-fold higher glycation level than M1 and had substantially elevated SBP and DBP values compared with M1. These results support the notion that more glycation in HDL was positively correlated with SBP and DBP as shown in Figure 5C,D and Figure 7A,D.
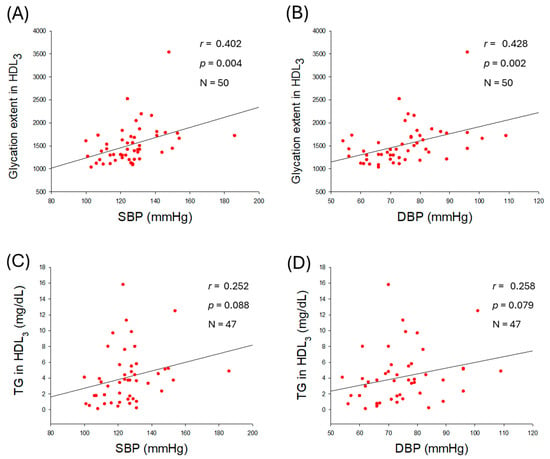
Figure 7.
Correlation analysis of high-density lipoprotein (HDL3) quality and blood pressure (BP). (A) Regression analysis of glycation extent in HDL3 with systolic blood pressure (SBP). (B) Regression analysis of glycation extent in HDL3 and diastolic blood pressure (DBP). (C,D) Regression analysis of triglyceride (TG) content in HDL3 with SBP and DBP, respectively; the data for TG is from only 47 participants, as data from 3 participants showed some inconsistency due to lower volume of HDL3.
2.8. Glycation Extent in HDL3 and Correlation with Blood Pressure
As shown in Figure 7, glycation extent in HDL3 was positively correlated with elevation of SBP (r = 0.402, p = 0.004) and DBP (r = 0.428, p = 0.002), while TG content in HDL3 was associated with non-significant (p > 0.05) elevation of BP. These results suggest that an increase in glycation extent in HDL3 is a reliable risk marker for hypertension.
2.9. Correlation of LDL Qualities and Antioxidant Abilities in HDL2
As shown in Figure 8A,B, the extent of LDL oxidation was negatively correlated with paraoxonase (PON) activity (r = −0.344, p = 0.014) and ferric ion reduction ability (FRA) in HDL2 (r = −0.320, p = 0.024), whereas HDL3-associated PON and FRA were not significantly correlated with the extent of LDL oxidation (Supplementary Figure S5A,B). Interestingly, no correlation was observed between the glycation extent of LDL and HDL2-associated PON (r = −0.015, p = 0.920) or FRA (r = −0.131, p = 0.364; Supplementary Figure S5C,D). However, a negative correlation was observed between the glycation extent of LDL and PON (r = −0.281, p = 0.048) activity associated with HDL3 (Figure 8C). However, the glycation extent in LDL was non-significantly associated with the FRA (r = −0.294, p = 0.038) activity of HDL3 (Figure 8D).
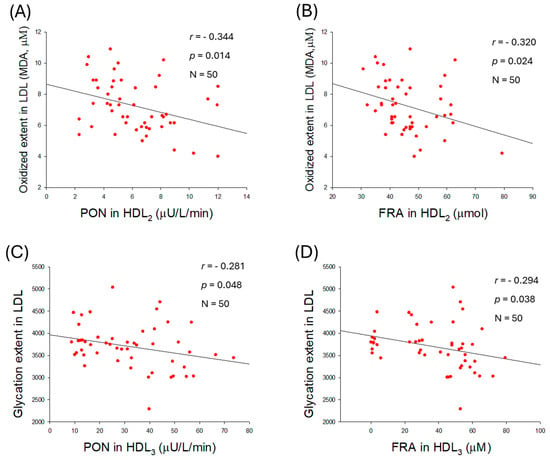
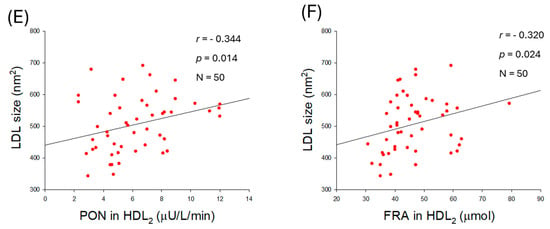
Figure 8.
Correlation analysis of low-density lipoprotein (LDL) quality and high-density lipoprotein (HDL)-associated antioxidant activities. (A) Regression analysis between oxidized extent in LDL and paraoxonase (PON) in HDL2. (B) Regression analysis between oxidized extent in LDL and ferric ion reduction (FRA) in HDL2. (C) Regression analysis between glycation extent in LDL and PON in HDL3. (D) Regression analysis between glycation extent in LDL and FRA in HDL3. (E) Regression analysis between LDL size and PON in HDL2. (F) Regression analysis between LDL size and FRA in HDL2.
Furthermore, an increase in LDL size was associated with an enhancement of HDL2-associated PON (r = −0.344, p = 0.014) and FRA (r = −0.320., p = 0.024) activities (Figure 8E,F). Nevertheless, no correlation of LDL size change was noticed on the HDL3-associated PON (r = 0.076, p = 0.599) and FRA (r = 0.004, p = 0.976) (Supplementary Figure S5E,F). Collectively, the results indicated that alterations in LDL, such as glycation, oxidation, and smaller particle size, are associated with the antioxidant activity of either HDL2 or HDL3.
2.10. Quality of HDL2 and Antioxidant Abilities of HDL2
The extent of glycation in HDL2 was negatively correlated with the enhancement of PON (r = −0.456, p < 0.001) and FRA (r = −0.340, p = 0.016) activities (Figure 9A,B). In addition, the particle size of HDL2 was positively associated only with PON activity (r = 0.388, p = 0.005) but was unrelated to FRA activity (r = 0.164, p = 0.255). The results indicated that higher PON activity is an important factor associated with reducing glycation and increasing HDL2 particle size.
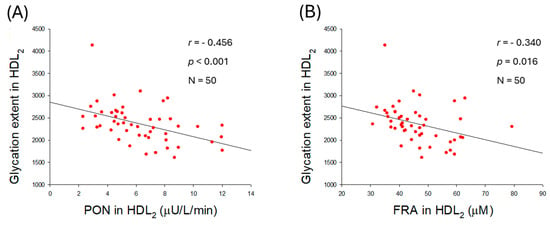
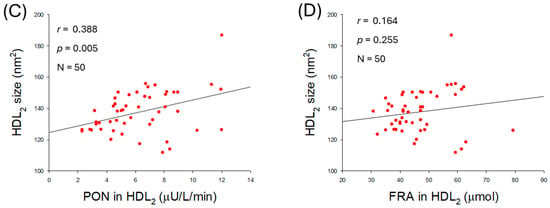
Figure 9.
Correlation analysis between the quality of high-density lipoprotein (HDL2) and antioxidant abilities of HDL2. (A) Regression analysis between glycation extent in HDL2 and paraoxonase (PON) in HDL2. (B) Regression analysis between glycation extent in HDL2 and ferric ion reduction (FRA) in HDL2. (C) Regression analysis between particle size of HDL2 and PON in HDL2. (D) Regression analysis between particle size of HDL2 and FRA in HDL2.
2.11. Correlation Analysis of Parameters Between SBP and DBP
As summarized in Table 2, BMI and visceral fat exhibited significant positive correlations with both SBP and DBP. In addition, serum TG, TG/HDL-C, glucose, and glucose/HDL-C levels were also significantly and positively correlated with both SBP and DBP. These results indicate that general obesity and abdominal obesity are important risk factors of hypertension, as elevated TG and glucose levels, along with reduced HDL-C, may contribute to the accumulation of visceral fat in the abdomen.
In LDL, TG content, oxidation extent, and glycated extent were positively correlated with both SBP and DBP, while particle size and diameter were negatively correlated with both SBP and DBP. These results suggest that more oxidized, glycated, TG-enriched, and smaller LDL is directly linked with the incidence of hypertension. In HDL2, TG content and glycation extent were positively correlated with both SBP and DBP, while particle size and diameter were negatively correlated with both SBP and DBP. In HDL3, only the extent of glycation was positively correlated with elevated SBP and DBP. These results suggest that higher TG content, greater glycation extent, and smaller HDL particle size are correlated with impairment of HDL functionality.
3. Discussion
The observational outcomes from the 50 participants, as summarized in Table 2, revealed that several anthropometric and lipid parameters were positively associated with higher BP. Specifically, BMI, visceral fat, serum TG, TG/HDL-C ratio, and serum glucose showed positive associations with increases in BP (Figure 1 and Figure 2). In contrast, serum HDL-C/TC (%) was negatively correlated only with SBP (r = −0298, p = 0.036), but not with DBP. In addition, weak correlations were observed between age, body fat percentage, subcutaneous fat (kg), and serum LDL-C, and HDL-C with SBP and DBP. These results are in good agreement with a previous report documenting a substantial effect, with the HDL-C/TC (%) ratio associated with a change in SBP, while SBP is well correlated with a decrease in HDL-C [24]. Specifically, women showed a sharper decrease in HDL-C, correlating with increasing SBP, after menopause, according to the Korean National Health and Nutrition Examination Survey in 2017 [24]. A decrease in HDL-C in the female group after middle age was strongly associated with a considerable increase in dementia in later life [25]. The study outcomes are in accordance with previous reports deciphering a deep association of elevated BMI [26] and visceral fat [27] with hypertension. Consistent with present findings, earlier reports showed the impact of dyslipidemia with hypertension [28]. In line with the current findings, a higher TG/HDL-C ratio emerged as a predictor of hypertension [29]. Although earlier reports demonstrated a positive association between TC levels and BP [28], our findings did not show a significant individual effect of TC or HDL-C on BP. Instead, HDL-C in relation to TC (i.e., HDL-C/TC ratio) was inversely associated with BP, highlighting its relevance as a possible predictor of hypertension risk.
In LDL, the increase in TG content was more strongly correlated with the elevation of SBP, with a higher regression coefficient (r = 0.506) than with DBP (r = 0.448) and higher significance (Figure 3A,B). The increase in oxidized LDL (Figure 3C,D) was more strongly correlated with DBP elevation, with a higher regression coefficient (r = 0.672) than with SBP (r = 0.617), suggesting that compositional changes in LDL, particularly with respect to TG and oxidation extent, are differently associated with SBP and DBP. Furthermore, as shown in Figure 4 and Table 2, the increase in glycation extent was more strongly associated with an elevation in DBP (r = 0.542) than with an elevation in SBP (r = 0.464), with greater significance. The decrease in LDL particle size was also associated with an elevation of DBP, with a higher regression coefficient (r = −0.501, p < 0.001) than with an elevation of SBP (r = −0.436, p = 0.002) and higher significance. Although the mechanism remains unclear, these results suggest that changes in lipid composition, oxidation extent, glycation extent, and LDL particle size were closely associated with SBP or DBP. Most of the reports depict glycated and oxidized LDL as an important marker of diabetes [30] and coronary heart disease [31,32], though its impact on hypertension is not highlighted much. However, the current observational study gives a preliminary indication of the relationship between elevated BP and glycated and oxidized LDL, highlighting its relevance beyond coronary heart disease.
Antioxidant activities of HDL were also negatively correlated with glycated extent; higher glycation content is associated with diminished PON and FRA activities in both HDL2 and HDL3 (Figure 9A,B). An increase in HDL2 particle size is significantly correlated with enhancement of only PON activity (r = 0.388, p = 0.005) in HDL2, not associated with FRA in HDL2 (r = 0.164, p = 0.255). Although LDL particle size was intimately associated with both PON and FRA activities in HDL2, HDL2 particle size was positively correlated with only higher PON activity, not FRA, in HDL2 itself. Collectively, larger HDL particles (both HDL2 and HDL3) exhibit stronger antioxidant activity, and correlate with protecting LDL (Figure 10). These findings align with the existing literature, which indicates that HDL quality holds greater significance than quantity in CVD risk assessment [33].
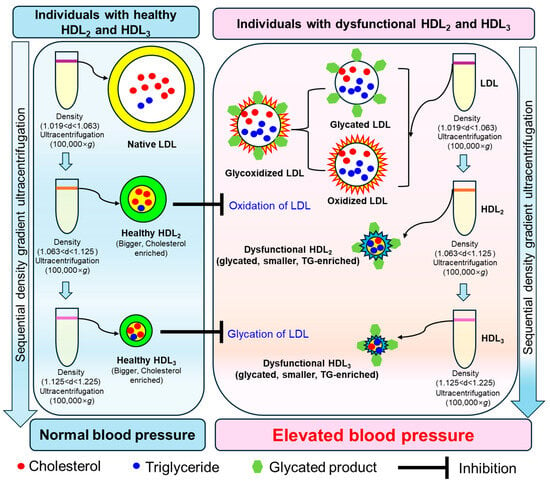
Figure 10.
This schematic illustration shows the association between low-density lipoprotein (LDL) and high-density lipoprotein (HDL) subpopulations (HDL2 and HDL3), including their quality, particle size, and extent of oxidation and glycation, with blood pressure, based on outcomes from a cross-sectional study.
On the other hand, higher TG content and greater HDL glycation were positively associated with elevated SBP and DBP, whereas HDL2 particle size was negatively correlated with SBP and DBP. These results suggest that TG-enriched, highly glycated, smaller HDL particles are associated with loss of apoA-I and an increased risk of CVD, rather than HDL cholesterol quantity [34,35,36,37]. More interestingly, PON and FRA activities in HDL2 were negatively correlated with oxidation extent in LDL, while an increase in LDL particle size was positively correlated with PON and FRA activity in HDL2 [38]. Furthermore, the extent of glycation in LDL was negatively correlated with PON and FRA activity in HDL3, suggesting distinct roles for HDL2 and HDL3 associated with LDL oxidation and glycation, respectively. Although a report showed that HDL3 exerts a more powerful antioxidative effect than HDL2 [39], another report showed that both HDL2 and HDL3 play an important role in the copper-catalyzed oxidation of LDL [40].
M3 and M4 have the same profession, as professional accountants, and the same age (51 years old), but they showed substantially different blood pressure. A comparison of HDL and LDL quality between the two peers revealed that LDL quality is correlated with HDL quality and functionality, as illustrated in Figure 4. LDL from M3 showed a round shape with distinct and separate distribution and larger particle size around 25.6 ± 2.2 nm. However, LDL from M4 showed a distorted shape, aggregation, and uneven distribution with a smaller particle size of around 24.4 ± 3.6 nm. These results suggest that the morphology of LDL particles, including shape, size, and number, differed between the two men, regardless of the quantity of LDL-C. Strikingly, HDL particles from M3 exhibited a well-defined spherical morphology, uniform dispersion, and a larger average particle size of 13.2 ± 0.9 nm with a higher particle count, as illustrated in Figure 6. In contrast, HDL particles from M4 displayed irregular shapes, aggregation tendencies, less particle numbers, and uneven distribution with smaller average particle size, around 12.2 ± 0.7 nm, with 1.3-fold higher glycation extent.
Although the precise role of HDL associated with glycation is not fully known, research shows that a key protective enzyme in HDL (i.e., PON) loses its activity when exposed to methylglyoxal, creating dysfunctional HDL that can no longer protect against oxidation and inflammation [41]. It is well established that both HDL2 and HDL3 are correlated with protecting LDL from damage; however, HDL2 is more specifically associated with protection from oxidation, while HDL3 is more correlated with minimizing glycation, as summarized in Figure 10.
We analyzed, in the current study, various aspects of HDL quality and functionality regarding its correlation with LDL and hypertension in middle-aged participants, especially pre-hypertension in male participants. Major findings of this study are that HDL quality and antioxidant ability are more correlated with the incidence of pre-hypertension than the simple quantity of HDL-C. A larger particle size, enriched cholesterol content, lower TG content, reduced glycation extent, and enhanced antioxidant activity are crucial for vascular health to protect against the incidence of hypertension.
This study includes multiple correlation analyses between the variables and is interpreted as explanatory and hypothesis-generating. The findings provided preliminary information that may be useful for future studies with a larger participant pool with prespecified models.
Limitations and prospects of this study: A small number of participants (n = 50) emerged as a basic limitation of this study to establish a confirmed relationship between HDL functionality, size, and LDL glycation and a risk of hypertension in the Korean population. In addition, the lack of detailed mechanistic insight limits the study’s outcomes. Future studies will focus on large participant pools and on decoding how lipoprotein glycation and antioxidant activities mechanistically lead to hypertension.
4. Materials and Methods
4.1. Participants
A total of 50 middle-aged volunteers (25 males aged 49.2 ± 11.7 years and 25 females aged 44.8 ± 11.5 years) were randomly recruited via a nationwide advertisement in Korea (2022 to 2023). The study aimed to assess HDL and LDL qualities and quantities. The protocol for human blood donation was conducted according to the latest version of Declaration of Helsinki [42]. The study received ethical approval from the Korea National Institute for Bioethics Policy (KoNIBP, approval number P01-202109-31-009, date of approval 27 September 2021) under the Ministry of Health Care and Welfare (MOHW).
The exclusion criteria were as follows: (1) Maintenance treatment for metabolic disorder, including severe dyslipidemia, hypertension, and diabetes. The exclusion criteria are specifically related to previously diagnosed participants who are receiving ongoing hypertensive treatment. However, newly identified, untreated hypertensive participants at the time of enrolment were included in the study. (2) Severe hepatic, renal, cardiac, respiratory, endocrinological, and metabolic disorder disease. (3) Allergies and autoimmune diseases. (4) Heavy drinkers, more than 30 g of alcohol per day. (5) Taking medicines that may affect lipid metabolism, including raising HDL-C or lowering LDL-C concentration, and lowering triglyceride concentration. (6) Current smoker. (7) Women in pregnancy, in lactation, or planning to become pregnant during the study period. (8) Persons who donated more than 200 mL of blood within one month or 400 mL of blood within three months before starting this study. (9) Others considered unsuitable for the study at the discretion of the principal investigator.
4.2. Anthropometric Analysis
The blood pressure (BP) was measured using an Omron HBP-9020 (Kyoto, Japan). Based on the measured BP, participants were categorized into three groups, namely normotensive (<120 mmHg DBP and <80 mmHg SBP), pre-hypertensive (120–139 mmHg DBP and 80–89 mmHg SBP), and hypertensive (>140 mmHg DBP and >90 mmHg SBP) [43]. The height, body weight, body mass index (BMI), body water, total body fat (%), total body fat mass (kg), and visceral fat mass (VFM) (kg) were measured individually using an X-scan plus II body composition analyzer (Jawon Medical, Gyeongsan, Republic of Korea).
4.3. Blood Analysis
After an overnight fast, blood was collected using a Vacutainer (BD BioSciences, Franklin Lakes, NJ, USA) without the addition of an anti-coagulant. The serum parameters, as shown in Table 1, were determined by an automatic analyzer (CobasC502; Roche, Germany) at a commercial diagnostic service via SCL Healthcare (Seoul, Republic of Korea). For all the blood analysis parameters, researchers were blinded to the allocated group. Samples were coded with a non-revealing numerical code prior to the analysis.
4.4. Isolation of Lipoproteins
Sequential density gradient ultracentrifugation was performed to isolate serum lipoprotein fractions LDL, HDL2, and HDL3 [44]. In brief, 5 mL of serum from each individual was separately mixed with a density gradient solution (d < 1.019 g/mL) and centrifuged at 100,000× g. After 24 h, the separated very low-density lipoprotein (VLDL) fraction (d < 1.019 g/mL) was removed, and the density of the remaining serum was adjusted to 1.019 < d < 1.063, with subsequent centrifugation at 100,000× g. Post 24 h, the LDL fraction (from the density zone 1.019 < d < 1.063) was recovered and stored in a separate tube. The residual serum density was adjusted to 1.063 < d < 1.125 for the isolation of HDL2 using ultracentrifugation (at 100,000× g for 24 h). The HDL2 fractions were recovered and kept in separate tubes, and residual serum density was adjusted to 1.125 < d < 1.225, followed by 24 h centrifugation at 100,000× g to isolate the HDL3. The density was adjusted with NaCl and NaBr using a previously described standard method [45]. The isolated lipoprotein fractions (LDL, HDL2, and HDL3) were individually dialyzed against Tris-buffered saline [TBS, 10 mM Tris-HCl, 140 mM NaCl, and 5 mM ethylene-diamine-tetraacetic acid (EDTA), pH 8.0]. All the samples were dialyzed for 24 h. The dialyzed LDL, HDL2, and HDL3 were recovered and stored in a refrigerator for further use.
The total TC and TG in the individual lipoprotein fractions (i.e., LDL, HDL2, and HDL3) were quantified using commercially available kits (cholesterol, T-CHO, and TG, Cleantech TS-S; Wako Pure Chemical, Osaka, Japan) following the manufacturer’s instructions. The protein concentrations of the lipoproteins were determined using the Lowry protein assay, as modified by Markwell et al. [46], with bovine serum albumin (BSA) as a standard.
4.5. Quantification of LDL Oxidation and Agarose Gel Electrophoresis
Oxidation in the isolated LDL was quantified by measuring the concentration of oxidized species using the thiobarbituric acid reactive substance (TBARS) assay [47]. In brief, 50 μL of the LDL fraction (1 mg/mL) was mixed with 50 μL of trichloroacetic acid (20%) and 100 μL of thiobarbituric acid (0.67%) and incubated at 95 °C for 10 min. Absorbance at 560 nm was recorded, and the results are expressed as formed malondialdehyde (μM).
In addition, oxidation of LDL was verified by agarose gel electrophoresis in the native state and the induced oxidative state (in vitro oxidation) [48]. For the in vitro oxidation, LDL (1 mg/mL, 50 μL) was mixed with CuSO4 (10 μM, 50 μL). The mixture was incubated at 37 °C for 30 min, and then subjected to agarose gel electrophoresis. A 0.5% agarose gel was prepared, and the samples (10 μL) from the native and in vitro-oxidized LDL were loaded in the individual wells. Electrophoresis was carried out using Tris–acetate–EDTA buffer (pH 8.0) at a constant voltage of 50 V [49]. After a sufficient run (~1 h), the gel was stained with 1.25% Coomassie Brilliant Blue (CBB) solution. The separated band intensities were quantified using ImageJ software (https://image.net/ij; accessed on 6 June 2025, version 1.53).
4.6. Paraoxonase Assay
The paraoxonase-1 (PON-1) activity in HDL2 and HDL3 was determined by measuring the hydrolysis of paraoxon to p-nitrophenol and diethylphosphate, using a previously described method [50]. In brief, 20 μL of HDL2 or HDL3 (1 mg/mL equivalent protein) was mixed with 180 μL Tris-HCl (90 mM) containing paraoxon-ethyl (0.6 M). Following 60 min incubation at 37 °C, absorbance at 415 nm was recorded. The PON-1 activity was then determined by measuring the initial velocity of p-nitrophenol production using the molar extinction coefficient (1.7 × 104/M/cm), and the PON activity is expressed as μU/L/min.
4.7. Ferric Ion Reducing Ability Assay
The ferric ion reducing ability (FRA) was determined using the method reported by Benzie and Strain [51]. Briefly, the FRA reagents were freshly prepared by mixing 20 mL of 0.2 M acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,6-tripyridyl-S-triazine, and 2.5 mL of 20 mM FeCl3·6H2O. The 20 μL of HDL2 or HDL3 (1 mg/mL equivalent protein) was mixed with 180 μL of freshly prepared FRA reagent. After 60 min of incubation at room temperature, the absorbance at 593 nm was recorded. The ferrous sulfate standard curve was prepared to quantify the results as FRA (μM, ferric equivalent).
4.8. Electrophoresis of HDL
Electrophoresis of HDL2 in the denatured state was carried out to compare apoA-I band intensities using SDS-PAGE. A 15% SDS-PAGE was prepared and 10 μL of HDL2 samples (1 mg/mL of protein) was loaded in the well. Electrophoresis was carried out at 50 V using the Tris–acetate–EDTA buffer (pH 8.0). After a sufficient run (~1 h), the gel was stained with 0.125% CBB, after which the relative band intensity of apoA-I was compared by band scanning using Gel Doc® XR (Bio-Rad, Hercules, CA, USA) and Quantity One software (version 4.5.2).
4.9. Glycation Extent of LDL, HDL2, and HDL3
The glycation extent of LDL, HDL2, and HDL3 was determined by reading the fluorometric intensity at 370 nm (excitation) and 440 nm (emission) employing a previously described method [52]. Glycation was measured in terms of the formed advanced glycation product (AGE) which gives the characteristic emission at 440 nm, while the exited one is at 370 nm. In brief, LDL, HDL2, and HDL3 were individually suspended with 500 μL of 0.2 M potassium phosphate–3 mM sodium azide buffer (pH 7.4) at a final concentration of 0.1 mg/mL (protein). Finally, the fluorescent intensity (FI) was examined at 370 nm (excitation) and 440 nm (emission) using the LS55 spectrofluorometer (PerkinElmer, Shelton, CT, USA), and results are depicted as glycation extent (AU, arbitrary unit).
4.10. Electron Microscopy
Transmission electron microscopy (TEM; Hitachi H-7800, Ibaraki, Japan) was performed at Raydel HDL Research Institute (Daegu, Republic of Korea) following a previously described method [44,53]. In brief, 5 μL of LDL/HDL2 samples (0.3 mg/mL in TBS) was mixed with 5 μL of 2% sodium phosphotungstate (PTA; pH 7.4) for the negative staining. Subsequently, 5 μL of the LDL/HDL2 suspension was placed on a formvar-coated 300-mesh copper grid and air-dried for 2 min. The excess suspension was blotted, and the grid was incubated at 50 °C for 2 h. Finally, the sample was visualized under TEM (HT-7800, Hitachi, Tokyo, Japan) at 100 kV accelerating voltage, and the images were captured at 150,000× magnification. For each sample, five independent replicates in five distinct formvar copper grids were prepared for TEM analysis, and images were captured.
Post TEM analysis, the captured images were analyzed using the Image J software (https://image.net/ij, accessed on 6 June 2025, version 1.53) for the determination of LDL/HDL2 particle size. For the quantification of particle size, 10 fields per image across the five independent replicates (i.e., 50 particles/sample) were analyzed, and results are depicted as mean value ± SD.
4.11. Data Analysis
All analyses in Table 1 were normalized by a homogeneity test of variances through Levene’s statistics. If not normalized, nonparametric statistics were used for the Kruskal–Wallis test. All values are expressed as the mean ± SD (standard deviation) for the continuous variables for the middle-aged group. All tests were two-tailed, and statistical significance was defined as p < 0.05.
Pearson’s correlation analysis was performed to determine the presence of a positive or negative association between the selected parameters. Statistical analyses were carried out using the SPSS statistical package version 28.0 (SPSS Inc., Chicago, IL, USA), incorporating sampling weights and adjusting for the complex survey design.
5. Conclusions
This cross-sectional study of 50 Korean participants revealed heightened serum TG and glucose levels associated with hypertension. An enhanced particle size of HDL (subpopulations, HDL2 and HDL3), with enhanced PON and FRA activities, shows an inverse correlation with LDL glycation, oxidative damage, and the risk of hypertension in Korean participants. Nevertheless, a comprehensive study with a large participant pool is needed to verify the current outcome and reach a confirmatory conclusion.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms27021108/s1.
Author Contributions
Conceptualization, K.-H.C.; methodology, C.-E.Y., S.H.L., Y.L., and A.B.; data curation, writing—original draft preparation, K.-H.C.; writing—review and editing, K.-H.C.; supervision, K.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol for human blood donation was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Korea National Institute for Bioethics Policy (KoNIBP, approval number P01-202109-31-009, date of approval 27 September 2021) and supported by the Ministry of Health Care and Welfare (MOHW) of Korea.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, X.P.; Zhao, S.P.; Zhang, X.Y.; Liu, L.; Gao, M.; Zhou, Q.C. Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int. J. Cardiol. 2000, 73, 231–236. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, L.; Nauman, E.; Katzmarzyk, P.T.; Price-Haywood, E.G.; Bazzano, A.N.; Nigam, S.; Hu, G. Inverse association between HDL (High-Density Lipoprotein) cholesterol and stroke risk among patients with type 2 diabetes mellitus. Stroke 2019, 50, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Taggart, H.; Stout, R.W. Reduced high density lipoprotein in stroke: Relationship with elevated triglyceride and hypertension. Eur. J. Clin. Investig. 1979, 9, 219–221. [Google Scholar] [CrossRef]
- Liu, X.; Tao, L.; Cao, K.; Wang, Z.; Chen, D.; Guo, J.; Zhu, H.; Yang, X.; Wang, Y.; Wang, J.; et al. Association of high-density lipoprotein with development of metabolic syndrome components: A five-year follow in adults. BMC Public Health 2015, 15, 412. [Google Scholar] [CrossRef]
- Yang, G.; Qian, T.; Sun, H.; Xu, Q.; Hou, X.; Hu, W.; Zhang, G.; Drummond, G.R.; Sobey, C.G.; Witting, P.K.; et al. Adjustment for body mass index changes inverse associations of HDL-cholesterol with blood pressure and hypertension to positive associations. J. Hum. Hypertens. 2022, 36, 570–579. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Kieneker, L.M.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. The inverse association of HDL-cholesterol with future risk of hypertension is not modified by its antioxidant constituent, paraoxonase-1: The PREVEND prospective cohort study. Atherosclerosis 2017, 263, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2022, 44, 1394–1407. [Google Scholar] [CrossRef]
- Ryu, H.E.; Jung, D.H.; Heo, S.J.; Park, B.; Lee, Y.J. Extremely high HDL cholesterol paradoxically increases the risk of all-cause mortality in non-diabetic males from the Korean population: Korean genome and epidemiology study-health examinees (KoGES-HEXA) cohorts. Front. Med. 2025, 12, 1534524. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arter. Thromb. Vasc. Biol. 2021, 41, 128–140. [Google Scholar] [CrossRef]
- Bonizzi, A.; Piuri, G.; Corsi, F.; Cazzola, R.; Mazzucchelli, S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines 2021, 9, 729. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Steinberg, F.M. Relationship Between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Sun, Y.V.; Quyyumi, A.A. Very High High-Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality. Am. J. Cardiol. 2022, 167, 43–53. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Rashid, S.; Watanabe, T.; Sakaue, T.; Lewis, G.F. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: The combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin. Biochem. 2003, 36, 421–429. [Google Scholar] [CrossRef]
- Smith, J.D. Dysfunctional HDL as a diagnostic and therapeutic target. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 151–155. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef]
- Sakata, N.; Uesugi, N.; Takebayashi, S.; Nagai, R.; Jono, T.; Horiuchi, S.; Takeya, M.; Itabe, H.; Takano, T.; Myint, T. Glycoxidation and lipid peroxidation of low-density lipoprotein can synergistically enhance atherogenesis. Cardiovasc. Res. 2001, 49, 466–475. [Google Scholar] [CrossRef]
- Younis, N.; Charlton-Menys, V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, S.; Lu, J.; Wu, M. Small, Dense Low-Density Lipoprotein-Cholesterol and Atherosclerosis: Relationship and Therapeutic Strategies. Front. Cardiovasc. Med. 2021, 8, 804214. [Google Scholar] [CrossRef]
- Hidaka, A.; Inoue, K.; Kutsukake, S.; Adachi, M.; Kakuta, Y.; Kojo, S. Decrease in the particle size of low-density lipoprotein (LDL) by oxidation. Bioorg. Med. Chem. Lett. 2005, 15, 2781–2785. [Google Scholar] [CrossRef]
- Bentley, A.R.; Rotimi, C.N. Interethnic variation in lipid profiles: Implications for underidentification of African–Americans at risk for metabolic disorders. Exp. Rev. Endocrinol. Metab. 2012, 7, 659–667. [Google Scholar] [CrossRef]
- Zakai, N.A.; Minnier, J.; Safford, M.M.; Koh, I.; Irvin, M.R.; Fazio, S.; Cushman, M.; Howard, V.J.; Pamir, N. Race-dependent association of high-density lipoprotein cholesterol levels with incident coronary artery disease. J. Am. Coll. Cardiol. 2022, 80, 2104–2115. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, H.-J.; Kim, J.-R. Decrease in serum HDL-C level is associated with elevation of blood pressure: Correlation analysis from the Korean National Health and nutrition examination survey 2017. Int. J. Environ. Res. Public Health 2020, 17, 1101. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, H.-J.; Kim, S.-J.; Kim, J.-R. Decrease in HDL-C is associated with age and household income in adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation analysis of low HDL-C and poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G.; et al. Body mass index is strongly associated with hypertension: Results from the longevity check-up 7+ study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Reang, T.; Sarkar, S.; Sengupta, S.; Bhattacharjee, B. Role of body visceral fat in hypertension and dyslipidemia among the diabetic and nondiabetic ethnic population of Tripura—A comparative study. J. Fam. Med. Prim. Care 2020, 9, 2885. [Google Scholar] [CrossRef]
- Deng, G.; Li, Y.; Cheng, W. Association of lipid levels with the prevalence of hypertension in Chinese women: A cross-sectional study based on 32 health Check centers. Front. Endocrinol. 2022, 13, 904237. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Lai, W.; Liu, J.; Zhou, B. Association between TG/HDL-C and hypertension in Chinese middle-aged and older adults: Findings from CHARLS. BMC Cardiovasc. Disord. 2025, 25, 254. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Virella, M.F.; Hunt, K.J.; Baker, N.L.; Lachin, J.; Nathan, D.M.; Virella, G. Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes 2011, 60, 582–589. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Toshima, S.; Hasegawa, A.; Kurabayashi, M.; Itabe, H.; Takano, T.; Sugano, J.; Shimamura, K.; Kimura, J.; Michishita, I.; Suzuki, T.; et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Bowden, R.G. Assessing high-density lipoprotein: Shifting focus from quantity to quality in cardiovascular disease risk assessment. Int. J. Transl. Med. 2024, 4, 369–380. [Google Scholar] [CrossRef]
- Lamarche, B.; Uffelman, K.D.; Carpentier, A.; Cohn, J.S.; Steiner, G.; Barrett, P.H.; Lewis, G.F. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J. Clin. Investig. 1999, 103, 1191–1199. [Google Scholar] [CrossRef]
- Barter, P.J. The causes and consequences of low levels of high-density lipoproteins in patients with diabetes. Diabetes Metab. J. 2011, 35, 101–106. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Y.; Fu, M.; Peng, T.; Liu, Y.; Long, S. The impact of plasma triglyceride and apolipoproteins concentrations on high-density lipoprotein subclasses distribution. Lipids Health Dis. 2011, 10, 17. [Google Scholar] [CrossRef]
- Amigó, N.; Torné, P.; Nordestgaard, L.T.; Di Giacomo-Barbagallo, F.; Merino, C.; Magni, P.; González-Lleó, A.; Andreychuk, N.; Catapano, A.L.; Masana, L.; et al. Triglycerides as Determinants of Global Lipoprotein Derangement: Implications for Cardiovascular Prevention. Int. J. Mol. Sci. 2025, 26, 8284. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sakuma, N.; Hibino, T.; Sato, T.; Fujinami, T. HDL3 exerts more powerful anti-oxidative, protective effects against copper-catalyzed LDL oxidation than HDL2. Clin. Biochem. 1997, 30, 221–225. [Google Scholar] [CrossRef]
- Huang, J.M.; Huang, Z.X.; Zhu, W. Mechanism of high-density lipoprotein subfractions inhibiting copper-catalyzed oxidation of low-density lipoprotein. Clin. Biochem. 1998, 31, 537–543. [Google Scholar] [CrossRef]
- Bacchetti, T.; Masciangelo, S.; Armeni, T.; Bicchiega, V.; Ferretti, G. Glycation of human high density lipoprotein by methylglyoxal: Effect on HDL-paraoxonase activity. Metabolism 2014, 63, 307–311. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects; World Medical Association: Helsinki, Finland, 2024. [Google Scholar]
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 2004. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf (accessed on 11 January 2026).
- Cho, K.H.; Nam, H.S.; Baek, S.H.; Kang, D.J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial effect of Cuban policosanol on blood pressure and serum lipoproteins accompanied with lowered glycated hemoglobin and enhanced high-density lipoprotein functionalities in a randomized, placebo-controlled, and double-blinded trial with healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Markwell, M.A.C.; Haas, S.M.; Biebar, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and in protein samples. Anal. Biochem 1978, 87, 206–211. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Morgan, J.; Leake, D. Oxidation of low density lipoprotein by iron or copper at acidic pH. J. Lipid Res. 1995, 36, 2504–2512. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef]
- Blatter Garin, M.-C.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.