Abstract
Blue light serves as a critical environmental cue regulating Glycine max (soybean) stem morphology, yet the hormonal mechanisms underlying varietal differences remain unclear. Previous studies have highlighted the role of blue light in modulating plant architecture, but the specific hormone interactions driving morphological divergence between soybean varieties remain underexplored. Two soybean varieties with contrasting stem phenotypes—Henong 60 (HN60, tall) and Heinong 48 (HN48, dwarf)—were subjected to 0% (full light) and 30% (shade) transmittance conditions, supplemented with blue light (450 nm, 45.07 ± 0.03 μmol·m−2·s−1). Stem anatomical traits (xylem area, cell length), hormone profiles, and proteomic changes were analyzed. Grey correlation analysis quantified relationships between hormone ratios and plant height. Blue light increased soybean stem xylem area and diameter while reducing plant height and cell longitudinal length. This treatment concurrently reduced growth-promoting hormones (gibberellin A3 (GA3), indole-3-acetic acid (IAA), brassinolide (BR)) and increased growth-inhibiting hormones (salicylic acid (SA), jasmonic acid (JA), strigolactones (SLs)), thereby inhibiting stem elongation. Although exogenous GA3 promoted hypocotyl elongation, it failed to counteract blue-light-induced inhibition. Proteomic analysis identified 16 differentially expressed proteins involved in hormone signal transduction pathways. Grey correlation analysis highlighted cultivar-specific hormone ratio impacts: GA3/JA, GA3/SA, and BR/SLs significantly influenced HN60 plant height, while GA3/SLs, IAA/SLs, and BR/SLs were critical for HN48, demonstrating highly significant positive correlations. The differential sensitivity of growth-promoting/inhibiting hormone ratios to blue light drives varietal morphological divergence in soybean stems. This study establishes a hormonal regulatory framework for blue-light-mediated stem architecture, offering insights for crop improvement under light-limited environments.
1. Introduction
Light serves as a critical environmental regulator governing plant morphogenesis and differentiation [1,2,3,4,5,6]. Among spectral components, blue light constitutes a pivotal photomorphogenetic signal exhibiting species-specific effects on developmental processes [7,8]. Physiological studies demonstrate that elevated blue light intensity reduces cucumber seedling growth and plant height through diminished cell wall extensibility [9,10]. This inhibitory mechanism contrasts with findings in Glycine max (soybean), where blue-light-mediated suppression of internode elongation primarily results from reduced epidermal cell dimensions and decreased cell density per internode unit [1,6,11].
Phytohormones function as essential signaling mediators in plant responses to spectral quality [12]. Gibberellins (GAs) and indole-3-acetic acid (IAA) coordinately regulate shade-avoidance responses and elongation growth [13,14,15]. Both reduced red/far-red ratios and low-intensity blue light environments elevate endogenous IAA and GA levels, thereby promoting stem elongation [16,17]. The inhibitory effect of blue light on petunia main stem elongation has been mechanistically linked to GA homeostasis modulation [18], while blue light exposure also influences abscisic acid (ABA), salicylic acid (SA), and IAA accumulation patterns [19]. Exogenous GA3, IAA, and 24-epibrassinolide application significantly enhances soybean hypocotyl elongation under white light, whereas biosynthetic inhibitors counteract shade-induced elongation, suggesting GA3 operates downstream of auxin and brassinosteroid signaling pathways [20]. Crucially, plant developmental outcomes emerge from hormonal crosstalk rather than isolated hormone actions. Ratios between hormonal groups, including GA3/IAA, GA3/cytokinins (CKs), and GA3/ABA, demonstrate regulatory significance in adventitious root formation in passion fruit cuttings [21]. Similarly, elevated IAA/ABA and CK/ABA ratios promote Pinus tabulaeformis vegetative growth [22], while reduced ABA/IAA ratio impairs tomato growth vigor [23]. Notably, the IAA/gibberellin A1 (GA1) ratio has been proposed as a key indicator of soybean photomorphogenetic responses, with far-red supplementation or reduced light intensity differentially modulating this ratio across leaf, petiole, and stem tissues [24]. However, current research predominantly focuses on individual hormone effects, with limited exploration of the synergistic/antagonistic relationships between hormonal ratios—a critical knowledge gap that this study addresses regarding blue light regulation of soybean stem elongation.
Advances in proteomics have illuminated plant photomorphogenetic mechanisms under varying light spectra. Comparative proteomic analysis revealed blue-light-induced suppression of potato seedling growth through differential regulation of hormone signaling proteins and concomitant GA3/IAA reduction [25]. Contrastingly, blue light enhances industrial hemp growth via auxin biosynthesis upregulation and starch accumulation [26]. Grey-system-theory-based correlation analysis provides a robust framework for multi-parameter optimization in complex biological systems [27]. While widely applied in crop studies evaluating abiotic stress responses [28,29,30,31,32,33,34], its potential in photomorphogenetic research remains underexplored. This study utilized two contrasting soybean cultivars, Henong 60 (HN60) and Heinong 48 (HN48), characterized by distinct morphological architectures and lodging resistance capacities, as experimental models. Employing integrated correlation and grey relational analyses, we systematically identified the core phytohormones governing soybean growth modulation under blue light. A multi-omics approach combining morphological phenotyping, histological characterization, hormonal quantification, gene expression profiling, and proteomic interrogation revealed coordinated hormonal networks underlying blue-light-mediated stem elongation dynamics. These findings establish a mechanistic framework for photomorphogenetic regulation in legumes, providing both theoretical insights into light-quality adaptation and potential applications for precision cultivation.
2. Results
Comparative analysis revealed distinct stem anatomical characteristics between treatments (Figure 1). Shade + blue light (SB) treatment plants exhibited enhanced stem diameter and xylem area compared to shading (S) treatment counterparts. Both treatments displayed progressive expansion of medullary cells from peripheral to central regions, with SB treatment specimens showing improved radial cell alignment and reduced longitudinal cell dimensions.
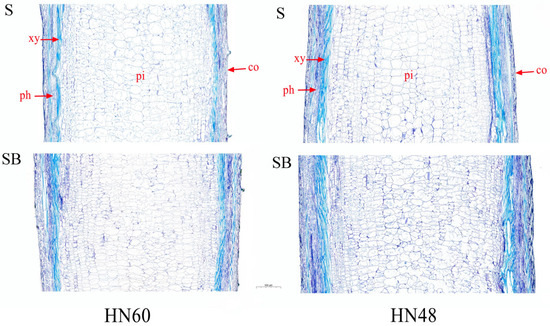
Figure 1.
Anatomical structure of stem longitudinal section under blue light treatment and under shading. HN60: Henong 60; HN48: Heinong 48. S: shading treatment; SB: shade + blue light. co: cortex; xy: xylem; ph: phloem; pi: pith. The scale is 500 μm.
Figure 2 demonstrates blue-light-mediated suppression of internode elongation, particularly evident in HN60′s fourth (44.42% reduction) and fifth internodes (34.32% reduction), contrasting with HN48′s broader inhibition across the third to sixth internodes (19.95–58.56% reduction). These findings indicate blue light’s dual regulatory role in stem thickening and longitudinal growth restriction.
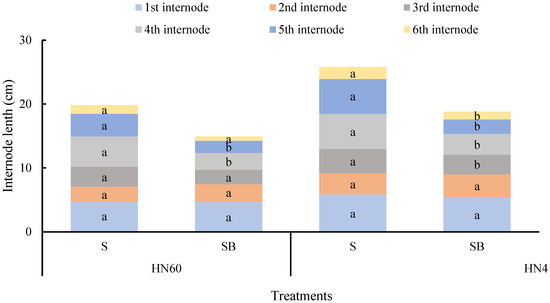
Figure 2.
Difference in internode length of soybean stem under blue light treatment and under shading (cm). HN60: Henong 60; HN48: Heinong 48. S: shading treatment; SB: shade + blue light. Means were compared using one-way ANOVA. Different letters in the same internode of each variety in the figure indicate significant differences between treatments (p < 0.05, n = 10). The measurements were taken from distinct samples (biological replicates).
Temporal analysis of hypocotyl elongation revealed biphasic growth patterns (Table 1). Both cultivars exhibited rapid initial elongation (0–3 days), followed by growth stabilization (3–7 days). Blue light significantly suppressed hypocotyl elongation in both varieties, with treatment differences reaching statistical significance from day 3 onward. Conversely, plant height increased by 64.02% (HN60) and 64.64% (HN48) under blue light, demonstrating spectral-quality-dependent growth modulation.

Table 1.
Differences in hypocotyl length and plant height under blue light treatment in darkness (cm).
Table 2 illustrates the significant impact of blue light on stem hormone profiles in both cultivars. Relative to the S treatment, the SB treatment induced a significant reduction in GA3 and IAA concentrations across the third, fourth, and fifth internodes of HN60 and HN48. Stem BR content was also significantly decreased under SB treatment, with the exception of the third internode, suggesting that blue light exposure suppresses growth-promoting hormone biosynthesis. Conversely, SB treatment resulted in marked increases in JA, SA, and SLs concentrations in all evaluated internodes, indicating a potential role of blue light in enhancing stress-responsive hormone accumulation.

Table 2.
Difference of stem hormone concentration under blue light treatment and under shading.
As presented in Table 3, compared to the darkness, BL treatment elicited significant reductions in GA3, IAA, and BR levels while promoting substantial increases in JA, SA, and SLs concentrations in both cultivars. These findings demonstrate that supplementary blue light under dark conditions can significantly modulate phytohormone concentrations, particularly by attenuating the accumulation of growth-promoting hormones GA3, IAA, and BR.

Table 3.
Differences in hypocotyl hormone concentrations under blue light treatment in darkness.
Analysis of Figure 3 reveals that relative to distilled water treatment, application of 100 mg L−1 GA3 significantly promoted soybean hypocotyl elongation, with the most pronounced changes occurring within 0–3 days post-treatment. Hypocotyl length under blue light conditions exceeded that observed in darkness, and HN48 exhibited more pronounced hypocotyl length variations compared to HN60. These results indicate that 100 mg L−1 GA3 effectively promotes hypocotyl elongation, yet fails to attenuate blue-light-induced inhibition of this process. Exogenous application of 100 mg L−1 IAA, 10 mg L−1 IAA, and 100 mg L−1 BR suppressed hypocotyl elongation in both cultivars under darkness. Notably, BR supplementation under blue light significantly augmented hypocotyl length in HN48 at 2, 3, and 4 days, whereas IAA had no measurable effect. HN48 demonstrated higher sensitivity to IAA, as reflected by greater hypocotyl length alterations.
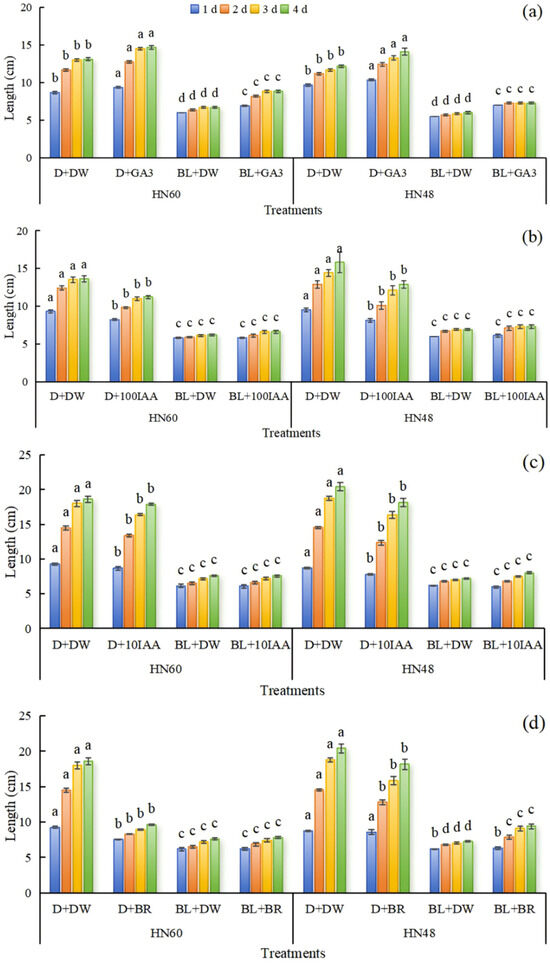
Figure 3.
Effects of exogenous GA3 (a), IAA (b,c), and BR (d) on hypocotyl length of soybean. HN60: Henong 60; HN48: Heinong 48. D + DW: darkness + distilled water; D + GA3: darkness + GA3; BL + DW: blue light + distilled water; BL + GA3: blue light + GA3; D + 100IAA: darkness + 100 mg L−1 IAA; BL + 100IAA: blue light + 100 mg L−1 IAA; D + 10IAA: darkness + 10 mg L−1 IAA; BL + 10IAA: blue light + 10 mg L−1 IAA; D + BR: darkness + BR; BL + BR: blue light + BR. D + DW: the hypocotyls were smeared with distilled water under dark conditions; D + GA3, D + 100IAA, D + 10IAA, and D + BR: the hypocotyls were smeared with 100 mg L−1 GA3, 100 mg L−1 IAA, 10 mg L−1 IAA, and 100 mg L−1 BR under dark conditions. BL + DW: the hypocotyls were smeared with distilled water under blue light conditions; BL + GA3, BL + 100IAA, BL + 10IAA, and BL + BR: the hypocotyls were smeared with 100 mg L−1 GA3, 100 mg L−1 IAA, 10 mg L−1 IAA, and 100 mg L−1 BR under blue light conditions. Means were compared using one-way ANOVA. Different letters in the same column of each variety on the same day indicated significant differences between treatments (p < 0.05, n = 6). The measurements were taken from distinct samples (biological replicates).
Plant hormone signaling pathways play a pivotal role in soybean stem development. This study identified 16 differential expressed proteins associated with phytohormone signal transduction under blue light treatment compared to darkness (Table 4). These proteins were involved in salicylic acid, gibberellin, jasmonic acid, and auxin metabolic and signal pathways. Blue light treatment upregulated nonexpressor of pathogenesis-related genes 1 (NPR1) in the SA signal pathway and acetyl-CoA acyltransferase 1 (ACAA1), lipoxygenase 1_5 (LOX1_5), lipoxygenase2S (LOX2S), and hydroperoxide lyase (HPL) in the jasmonic acid metabolic pathway, while downregulating gibberellin receptor GA-INSENSITIVE DWARF 1 (GID1C), allene oxide cyclase (AOC), and allene oxide synthase (AOS) in jasmonic acid metabolism and aldehyde dehydrogenase (ALDH) in auxin metabolism (Table 4).

Table 4.
Differential proteins involved in plant hormone metabolism and signal transduction under blue light.
Figure 4 illustrates significant negative correlations between HN60 plant height and GA3/IAA and BR/IAA ratios, alongside significant positive correlations with GA3/SA, GA3/JA, GA3/SLs, IAA/GA3, IAA/BR, IAA/SA, IAA/JA, IAA/SLs, BR/SA, BR/JA, and BR/SLs ratios. HN48 exhibited negative correlations with GA3/BR and extreme negative correlations with IAA/BR, while showing extreme positive correlations with GA3/SA, GA3/JA, GA3/SLs, IAA/SA, IAA/JA, IAA/SLs, BR/IAA, BR/SA, BR/JA, and BR/SLs. These results highlight the close association between plant height and hormone ratios in both cultivars.
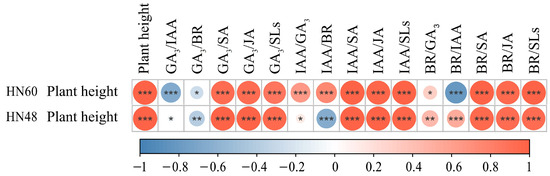
Figure 4.
Correlation between plant height and hormone ratio. HN60: Henong 60; HN48: Heinong 48. *: significant difference at the 0.05 level; **: significant difference at the 0.01 level; ***: significant difference at the 0.001 level.
Grey correlation analysis, shown in Figure 5, identifies the top three indices most strongly correlated with HN60 plant height as GA3/JA, GA3/SA, and BR/SLs, whereas GA3/IAA, BR/IAA, and GA3/BR showed weaker correlations. For HN48, the highest correlations were observed with IAA/SLs, GA3/SLs, and BR/SLs, while IAA/BR, GA3/BR, and BR/SA displayed lower associations. These findings underscore the differential effects of hormone interactions on plant height and significant cultivar-specific responses.
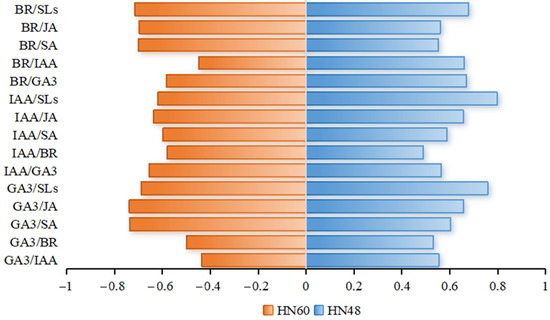
Figure 5.
Correlation degree. HN60: Henong 60; HN48: Heinong 48. The number in the figure indicates the correlation degree.
3. Discussion
Shading promotes internode elongation through modifications to soybean stem anatomical structure [35]. Liu et al. [36] reported that shading significantly reduced stem xylem area, pith area, and xylem proportion while increasing pith proportion in soybean, consistent with the findings of Wen et al. [37], who observed reduced xylem and pith areas under intensified shading. In tomato seedlings, Falcioni et al. [19] demonstrated that blue light increased cortical parenchyma cell thickness and number while decreasing xylem cell count compared to white light. Our results align with these observations, showing that blue light increased stem xylem area but reduced cell longitudinal length, thereby inhibiting soybean hypocotyl elongation, as previously reported by Wang et al. [6]. Cryptochrome-mediated blue light signaling is known to suppress hypocotyl elongation [38,39], with Arabidopsis cry1-304 mutants displaying exaggerated hypocotyl growth under blue light [40]. The basic leucine zipper (bZIP) transcription factor ELONGATED HYPOCOTYL 5 (HY5), a key photomorphogenesis regulator [41], was shown by Alabadi et al. [42] to interact with GAs signaling pathways. Our study detected upregulated cry1a and HY5 expression under blue light (Figure S1), suggesting their involvement in mediating hypocotyl growth responses in soybean.
A diverse array of phytohormones participate in the shade avoidance signaling cascade. Under shading stress, soybean stems exhibit reduced ABA and zeatin concentrations, concurrent with elevated IAA and GA3 levels. Increasing shading intensity correlates with enhanced internode elongation, accompanied by rising GA3 and SA contents and decreasing ABA concentration [3,43]. These trends align with the current study’s observations. Blue light treatment significantly reduces growth-promoting hormones—GA3, IAA, and BR—while augmenting stress-responsive hormones—SA, JA, and SLs. Phytochrome-interacting factors (PIFs) act as negative regulators of photomorphogenesis and serve as critical integrators between light and hormonal signaling pathways [44]. Hornitschek et al. [45] demonstrated that phytochrome-interacting factor 4 (PIF4) and phytochrome-interacting factor 5 (PIF5) modulate Arabidopsis seedling growth through direct regulation of auxin biosynthesis genes and signaling components. In this study, blue light exposure downregulated the relative expression levels of PIF4A and PIF4B (Figure S1), suggesting that blue light may influence hormone concentrations via transcriptional regulation of PIFs.
GA3 plays a pivotal role in regulating cell elongation [46], specifically governing the longitudinal cell expansion [47]. The soluble receptor GA-INSENSITIVE DWARF1 (GID1) perceives gibberellin signals [48], while DELLA proteins act as repressors of the gibberellin signaling pathway [49]. In this study, blue light exposure downregulated the gibberellin receptor protein GID1c (Table 4). Concurrent with this, DELLA gene expression and GA catabolic gene GA2ox4 were upregulated, whereas GA biosynthetic gene GA20OX1 was downregulated (Figure S1), aligning with findings from Zhao et al. [50]. These results indicate that blue light inhibits GA biosynthesis and promotes its degradation, thereby suppressing hypocotyl elongation. Exogenous application of 100 mg L−1 GA3 under darkness maximized soybean hypocotyl elongation. However, while GA3 supplementation under blue light increased hypocotyl length compared to blue light alone, it remained shorter than dark-grown controls (Figure 3), confirming that GA3 cannot fully reverse blue-light-induced growth inhibition. IAA, essential for plant elongation and development [51], is synthesized from indole-3-acetylaldehyde via aldehyde dehydrogenase (ALDH) in Escherichia coli [52]. Here, downregulation of ALDH in the tryptophan metabolic pathway (Table 4) suggests impaired IAA biosynthesis under blue light. Yang et al. [53] reported dose- and time-dependent stem elongation in pea seedlings treated with exogenous IAA. In contrast, our study observed that 10 mg L−1 and 100 mg L−1 IAA significantly inhibited soybean hypocotyl growth in darkness. While IAA-treated hypocotyls under blue light showed marginal increases compared to blue light alone, these differences were not statistically significant (Figure 3), highlighting species-specific responses to IAA and the need for further dose-response investigations under blue light. BR is a key regulator of plant elongation [54]. Exogenous BR promotes stem and leaf elongation in sweet pepper [55] but inhibits growth in Sophora davidii at high concentrations (50 mg L−1) [56]. Our results corroborate this duality: 100 mg L−1 BR suppressed hypocotyl growth in darkness but enhanced elongation under blue light (Figure 3), indicating that BR-mediated growth regulation is dose- and light-dependent.
Additionally, SA inhibits Arabidopsis hypocotyl elongation, with NPR1 serving as the central regulator of SA signaling [57]. Blue light exposure upregulated NPR1 protein abundance and increased SA content in this study. Lipoxygenase (LOX), the first committed enzyme in JA biosynthesis, initiates JA production by catalyzing the oxygenation of polyunsaturated fatty acids to linoleic and linolenic acids [58]. HPL, a downstream enzyme in the LOX-dependent lipid oxidation pathway, cleaves LOX-generated hydroperoxides into short-chain aldehydes and ω-oxo-acids [59]. ACAA1 participates in fatty acid metabolism by catalyzing the final step of the β-oxidation pathway, contributing to fatty acid elongation and degradation [60]. Upregulation of LOX, HPL, and ACAA1 under blue light treatment in this study suggests enhanced JA biosynthesis.
Phytohormone interactions and homeostasis play critical roles in plant morphogenesis and development. Wang et al. [61] reported that higher IAA/GA3 in peony (Paeonia suffruticosa) roots promoted adventitious root induction and elongation. Hou et al. [62] demonstrated that IAA/ABA and IAA/CTK ratios positively correlate with rooting efficiency in Chinese chestnut (Microshoots of Castanea mollissima). Similarly, Quan et al. [63] found that increased IAA/ABA and IAA/ZR ratios facilitated root primordium formation and early rooting in Catalpa bignonioides cuttings. Collectively, these studies highlight the context-dependent regulatory effects of hormone interactions on plant growth. In this study, grey correlation analysis revealed cultivar-specific hormone ratio impacts on plant height. GA3/JA, GA3/SA, and BR/SLs ratios exerted significant effects on HN60, whereas IAA/SLs, GA3/SLs, and BR/SLs ratios were critical for HN48. Blue light significantly reduced these key ratios in both cultivars (Table S1), indicating that differential balances between growth-promoting and growth-inhibiting hormones contribute to morphological divergence.
4. Materials and Methods
4.1. Experimental Design
Two soybean cultivars with contrasting lodging resistance and morphological traits were selected: Henong 60 (dwarf lodging-resistant variety) and Heinong 48 (high-stalk lodging-susceptible variety).
4.1.1. Experiment I: Shading and Blue Light Supplementation
A pot experiment was conducted using cylindrical pots (33 cm diameter × 30 cm height, 1 cm drainage hole diameter) filled with 15 kg soil. Fertilization included 1.5 g·pot−1 diammonium hydrogen phosphate (N: 18%, P2O5: 46%) and 0.75 g·pot−1 potassium sulfate (K2O: 50%). Seeds were sown in a single row along the pot diameter (8 holes·pot−1, 2 seeds·hole−1), thinned to 4 seedlings·pot−1. Treatments were applied at the V3 growth stage until V5:
S: shading with a black net (30% light transmittance).
SB: shading + supplementary blue light (LED tubes, 12 W, uniform lamp bead arrangement).
LED tubes were positioned 1 cm above the soybean canopy and adjusted vertically as plants grew. Irradiation occurred continuously from sunrise to sunset. At harvest, internode length and plant height were measured. Stem segments (third, fourth, fifth internodes) were rinsed with PBS buffer, wrapped in aluminum foil, snap-frozen in liquid nitrogen, and stored at −80 °C for hormone analysis. Anatomical observations of the third internode were performed.
4.1.2. Experiment II: Controlled Environment Chamber Study
A closed light chamber (120 cm × 60 cm × 180 cm) was used with 9 cm diameter × 10 cm height pots filled with flower nutrient soil. Uniform seeds were sown in three holes·pot−1 (2 seeds·hole−1), thinned to 1 seedling·pot−1. Seedlings were grown in darkness at 25 °C until hypocotyls reached ~5 cm. Blue light treatment (LED tube, 45.07 ± 0.03 μmol·m−2·s−1) was applied unilaterally 1 cm from the stem. During the whole experiment, the temperature of the incubator was maintained at 25 ± 1 °C, and the relative humidity was controlled at 50 ± 5%. Hypocotyl length and plant height were measured at 1, 3, 5, and 7 days post-treatment. Hypocotyl segments were collected at 3 days for hormone quantification, gene expression, and proteomic analysis. Each treatment was repeated three times.
4.1.3. Experiment III: Exogenous Hormone Treatments
Under the same sowing and growth conditions as Experiment II, the seedlings were cultured to about 5 cm hypocotyls at 25 °C in the dark, and then the soybean hypocotyls were treated with light and exogenous hormones. The specific settings are as follows: (1) Four treatments: darkness + distilled water, darkness + GA3, blue light + distilled water, and blue light + GA3. The concentration of GA3 solution (GA3 purity HPLC ≥ 90.0%, purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China)) was 100 mg L−1. (2) Four treatments: darkness + distilled water, darkness + IAA, blue light + distilled water, and blue light + IAA. The concentration of IAA solution (IAA purity ≥ 98%, purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China)) was 100 mg L−1 and 10 mg L−1. (3) Four treatments: darkness + distilled water, darkness + BR, blue light + distilled water, blue light + BR 4 treatments, in which BR solution (24-epibrassinolide purity ≥ 90%, purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China)). The solution concentration was 100 mg L−1. Each treatment received 1 mL solution. Hypocotyl length was measured at 0, 1, 2, 3, and 4 days. Light treatment parameters were identical to Experiment II.
4.2. Determination Indicators and Methods
4.2.1. Determination of Internode Length and Plant Height
The length of each internode of soybean stem and the length from soybean cotyledon scar to growth point were measured using a ruler.
4.2.2. Observation of Anatomical Structure
Soybean plants of uniform growth were carefully selected. Using a sharp blade, the middle part of the stem was cut into 5 mm segments. These segments were promptly placed into a brown bottle filled with FAA fixative. A vacuum pump was employed to evacuate the air from the samples until they sank to the bottom of the container. Subsequently, the fixed samples underwent a series of treatments. First, they were dehydrated to remove moisture and then made transparent to facilitate better visualization. The sections were then stained with toluidine blue. After staining, the samples were embedded in paraffin wax. The wax-embedded samples were sliced into thin sections, which were then mounted onto glass slides. The slides were baked to ensure proper adhesion of the sections. Next, the paraffin wax was removed through a dewaxing process. After dewaxing, the sections were re-stained and finally sealed to preserve the samples. The prepared sections were observed under a positive optical microscope (Nikon Eclipse E100). An imaging system (Nikon DS-U3) was used to capture high-quality photographs of the sections for further analysis.
4.2.3. Hormone Concentration Quantification
The concentrations of GA3, IAA, BR, JA, SA, and SLs were measured using enzyme-linked immunosorbent assay (ELISA) kits (purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China)). Briefly, 0.1 g of tissue samples was homogenized in 0.9 mL phosphate buffer solution (pH 7.4). After centrifugation at 12,000× g for 15 min at 4 °C, supernatants were collected. The ELISA protocol followed the manufacturer’s instructions: sample addition → enzyme conjugate addition → incubation (37 °C, 30 min) → washing (×5) → chromogenic substrate addition (37 °C, 15 min) → stop solution addition. Absorbance was measured at 450 nm using a microplate reader.
4.2.4. Detection of Real-Time Quantitative PCR Analysis
In this experiment, Actin served as the internal reference gene, and gene-specific primers were designed using Primer Premier 5.0 software. Relative expression levels of photoreceptors, phytochrome-interaction factors, HY5 transcription factor, gibberellin synthesis and decomposition gene, and DELLA protein gene were quantified by real-time quantitative polymerase chain reaction (RT-qPCR). The detailed detection steps are described in Supplementary Material S1.
4.2.5. Proteomic Analysis
After 3 days of dark and blue light treatment, 3 g of soybean hypocotyls were collected and divided into three parts, rinsed with PBS buffer, wrapped in aluminum foil, snap-frozen in liquid nitrogen, and stored at −80 °C. Proteome analysis was performed by Shanghai Applied Protein Technology Co. Ltd. according to the method of Wang et al. [5]. Three technical replicates were included for each sample. Specific steps are outlined in Supplementary Material S2.
4.2.6. Correlation Analysis
Pearson correlation coefficient was used to calculate the correlation between plant height and hormone ratio of the two varieties, and R Studio (https://posit.co/download/rstudio-desktop/, accessed on 28 April 2025) was used for mapping.
4.2.7. Grey Correlation Analysis Model
Firstly, the data are dimensionlessly processed according to Formulas (1) and (2):
In the formula, Yi is the mean value sequence of each sub-factor; Xi (k) is a sub-factor sequence, where i denotes the hormone ratio (i = 1, 2, 3, …, 16); and k is the number of data (k = 1, 2, 3, …, 18).
In the formula, Yo is the mean value sequence of the parent factor; Xo (k) is the maternal factor sequence, that is, soybean plant height; and k is the number of data (k = 1, 2, 3, …, 18).
The calculation of correlation coefficient (k) is as follows: the number of parent factors is listed as {Yo (k)}, and the number of sub-factors is listed as {Yi (k)}. In k, the grey correlation coefficient between {Yi (k)} and {Yo (k)} is:
In the formula, the absolute difference of two sequences at k points, that is, = |Yo (k) − Yi (k)|; Δmin and Δmax are the maximum and minimum values of absolute difference, respectively; and ρ is the resolution coefficient, which is 0.5 in most cases.
The calculation formula of grey correlation degree (γoi) is as follows:
4.3. Statistical Analysis
Data analysis and graphs were performed using SPSS 21.0 and Microsoft Excel 2010 software. The proteins with significantly different expression were screened according to the fold change > 1.2 times (upregulation by more than 1.2 fold or downregulation of less than 0.83 fold) and p-value < 0.05 (t-test) [5].
5. Conclusions
Blue light treatment promoted soybean stem thickening by increasing xylem area and reducing cell longitudinal length. This treatment also reduced growth-promoting hormones (GA3, IAA, and BR), and increased growth-inhibiting hormones (SA, JA, and SLs), thereby inhibiting stem internode elongation and reducing plant height. Proteomics analysis identified 16 differentially expressed proteins involved in salicylic acid, gibberellin, jasmonic acid, and auxin metabolic and signaling pathways. Exogenous GA3 promoted hypocotyl elongation in darkness but failed to alleviate blue-light-induced inhibition. Exogenous IAA and BR suppressed hypocotyl growth in darkness but had minimal effects under blue light. Grey correlation analysis revealed cultivar-specific hormone ratio impacts: GA3/JA, GA3/SA, and BR/SLs were strongly positively correlated with HN60 plant height, while GA3/SLs, IAA/SLs, and BR/SLs were critical for HN48. These results suggest that differential balances between growth-promoting and growth-inhibiting hormones contribute to morphological divergence between the two soybean varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26094411/s1.
Author Contributions
C.W., S.H. and F.S. performed the experiments. B.Y. collected the samples. X.L. and C.Y. conceived and planned the study. C.W. completed the paper writing. We thank C.M. and B.J. for overseeing and enhancing the writing process. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28010200) and the Agriculture Research System of China (CARS-04-PS22).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
It is declared that the authors do not have any competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| GAs | Gibberellins |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| BR | Brassinolide |
| SLs | Strigolactone |
| IAA | Indol-3-acetic acid |
| R | Red light |
| FR | Far-red light |
| ABA | Abscisic acid |
| CK | Cytokinin |
| LED | Light-emitting diode |
| PBS | Phosphate buffer saline |
| ELISA | Enzyme-linked immunosorbent assay |
| HY5 | ELONGATED HYPOCOTYL 5 |
| NPR1 | Nonexpressor of pathogenesis-related genes 1 |
| ACAA1 | Acetyl-CoA acyltransferase 1 |
| LOX | Lipoxygenase |
| HPL | Hydroperoxide lyase |
| GID1 | GA-INSENSITIVE DWARF 1 |
| AOC | Allene oxide cyclase |
| AOS | Allene oxide synthase |
| ALDH | Aldehyde dehydrogenase |
| PIFs | Phytochrome-interacting factors |
References
- Xu, Y.; Wang, C.; Zhang, R.; Ma, C.M.; Dong, S.K.; Gong, Z.P. The relationship between internode elongation of soybean stems and spectral distribution of light in the canopy under different plant densities. Plant Prod. Sci. 2020, 24, 326–338. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.Q.; Kaiser, E.; Kuang, Y.; Yang, Q.C.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Zhang, R.; Shan, F.X.; Wang, C.; Yang, C.; Dong, S.K.; Xu, Y.; Gong, Z.P.; Ma, C.M. Internode elongation pattern, internode diameter and hormone changes in soybean (Glycine max) under different shading conditions. Crop Pasture Sci. 2020, 71, 679–688. [Google Scholar] [CrossRef]
- Du, F.; Zou, Y.J.; Hu, Q.X.; Zhang, H.Y.; Ye, D. Comparative transcriptomic analysis reveals molecular processes involved in pileus morphogenesis in Pleurotus eryngii under different light conditions. Genomics 2020, 112, 1707–1715. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, H.; Chen, Y.; Xu, Y.; Shan, F.X.; Li, H.Y.; Yan, C.; Ma, C.M. Red light regulates metabolic pathways of soybean hypocotyl elongation and thickening. Environ. Exp. Bot. 2022, 199, 104890. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Cui, C.; Shan, F.X.; Zhang, R.; Lyu, X.C.; Lyu, L.; Chang, H.W.; Yan, C.; Ma, C.M. Blue light regulates cell wall structure and carbohydrate metabolism of soybean hypocotyl. Int. J. Mol. Sci. 2023, 24, 1017. [Google Scholar] [CrossRef]
- Li, H.M.; Xu, Z.G.; Tang, C.M. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Piszczek, P.; Głowacka, B. Effect of the colour of light on cucumber (Cucumis sativus L.) seedlings. J. Fruit Ornam. Plant Res. 2008, 68, 71–80. [Google Scholar] [CrossRef]
- Dougher, T.A.O.; Bugbee, B. Long-term blue light effects on the histology of lettuce and soybean leaves and stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Green, P.B. Mechanism of rapid suppression of cell expansion in cucumber hypocotyls after blue-light irradiation. Planta 1988, 176, 109–116. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Galvao, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Lorrain, S.; Fankhauser, C. Auxin-mediated plant architectural changes in response to shade and high temperature. Physiol. Plamtarum 2014, 151, 13–24. [Google Scholar] [CrossRef]
- Nozue, K.; Tat, A.V.; Devisetty, U.K.; Robinson, M.; Mumbach, M.R.; Ichihashi, Y.; Lekkala, S.; Maloof, J.N. Shade avoidance components and pathways in adult plants revealed by phenotypic profiling. PLoS Genet. 2015, 11, e1004953. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Farrow, S.; Walton, L.J.; Pharis, R.P.; Neil Emery, R.J.; Chinnappa, C.C. Phenotypic plasticity of sun and shade ecotypes of Stellaria longipes in response to light quality signaling, gibberellins and auxin. Plant Physiol. Biochem. 2015, 94, 174–180. [Google Scholar] [CrossRef]
- Lyu, X.G.; Cheng, Q.C.; Qin, C.; Li, Y.H.; Xu, X.Y.; Ji, R.H.; Mu, R.L.; Li, H.Y.; Zhao, T.; Liu, J.; et al. GmCRY1s modulate gibberellin metabolism to regulate soybean shade avoidance in response to reduced blue light. Mol. Plant 2021, 14, 298–314. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munne-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Ruggeberg, M.; et al. Cell wall structure and composition is affected by light quality in tomato seedlings. J. Photochem. Photobiol. B Biol. 2020, 203, 111745. [Google Scholar] [CrossRef]
- Jiang, H.K.; Shui, Z.W.; Xu, L.; Yang, H.Y.; Li, Y.; Yuan, X.Q.; Shang, J.; Asghar, M.A.; Wu, X.L.; Yu, L.; et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef]
- Khai, H.D.; Hiep, P.P.M.; Tung, H.T.; Phong, H.T.; Mai, N.T.N.; Luan, V.Q.; Cuong, D.M.; Vinh, B.V.T. Selenium nanoparticles promote adventitious rooting without callus formation at the base of passion fruit cuttings via hormonal homeostasis changes. Sci. Hortic. 2024, 323, 112485. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, L.H.; Ma, L.J.; Li, Y.Y. Elevated carbon dioxide and/or ozone concentrations induce hormonal changes in Pinus tabulaeformis. J. Chem. Ecol. 2011, 37, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Bettini, P.; Baraldi, R.; Rapparini, F.; Melani, L.; Mauro, M.L.; Bindi, D.; Buiatti, M. The insertion of the Agrobacterium rhizogenes rolC gene in tomato (Solanum lycopersicum L.) affects plant architecture and endogenous auxin and abscisic acid levels. Sci. Hortic. 2020, 123, 323–328. [Google Scholar] [CrossRef]
- Yang, F.; Fan, Y.F.; Wu, X.L.; Cheng, Y.J.; Liu, Q.L.; Feng, L.Y.; Chen, J.X.; Wang, Z.L.; Wang, X.C.; Yong, T.W.; et al. Auxin-to-gibberellin ratio as a signal for light intensity and quality in regulating soybean growth and matter partitioning. Front. Plant Sci. 2018, 9, 56. [Google Scholar] [CrossRef]
- Xu, J.M.; Liu, Y.; Liu, M.X.; Xu, Z.G. Proteomic, physiological, and anatomical analyses reveal the effects of red, blue, and white light on the growth of potato plantlets under in vitro culture. Russ. J. Plant Physiol. 2022, 69, 139. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, K.Y.; He, X.R.; Zheng, H.J.; Wang, R.; Liu, C.; Zhou, L.J.; Fahad, S.; Deng, G. Differential protein response to different light quality conditions of industrial hemp cultivation based on DIA technology. Ind. Crops Prod. 2023, 197, 116650. [Google Scholar] [CrossRef]
- Patil, A.N.; Walke, G.A.; Gawkhare, M. Grey relation analysis methodology and its application. Res. Rev. Int. J. Multidiscip. 2019, 4, 409–411. [Google Scholar]
- Ning, X.W.; Dong, P.P.; Wu, C.L.; Wang, Y.L.; Zhang, Y. Influence mechanisms of dynamic changes intemperature, precipitation, sunshine duration and active accumulated temperature on soybean resources: A case study of Hulunbuir, China, from 1951 to 2019. Energies 2022, 15, 8347. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.M.; Zhou, Q.; Song, S.; Dong, S.K. Comparison and evaluation of low-temperature tolerance of different soybean cultivars during the early-growth stage. Agronomy 2023, 13, 1716. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, S.M.; Jin, J.L.; Feng, P.; Ning, S.W. Decision-making of irrigation scheme for soybeans in the Huaibei Plain based on grey entropy weight and grey relation–projection pursuit. Entropy 2019, 21, 877. [Google Scholar] [CrossRef]
- Qiao, M.M.; Xia, G.Y.; Cui, T.; Xu, Y.; Gao, X.J.; Su, Y.; Fan, H.F. Effect of moisture, protein, starch, soluble sugar contents and microstructure on mechanical properties of maize kernels. Food Chem. 2022, 379, 132147. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Xu, Y.F.; Liu, H.G.; Li, C.; Yang, Y.; Zhao, P. Effect of different boron levels on yield and nutrient content of wheat based on grey relational degree analysis. Acta Physiol. Plant. 2021, 43, 127. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.T.; Yu, J.L.; Zhou, Y.F.; Wu, Q.; Gao, Y.; Xu, W.J.; Huang, R.D. Prioritization of feasible physiological parameters in drought tolerance evaluation in sorghum: A grey relational analysis. Zemdirb. Agric. 2015, 102, 457–464. [Google Scholar] [CrossRef][Green Version]
- Na, W.; Guan, Y.S.; Yan, S. Drought-resistance index in rice backcross lines after anthesis. Pak. J. Biol. Sci. 2007, 10, 2659–2664. [Google Scholar]
- Hussain, S.; Iqbal, N.; Pang, T.; Khan, M.N.; Liu, W.G.; Yang, W.Y. Weak stem under shade reveals the lignin reduction behavior. J. Integr. Agric. 2018, 17, 60345–60347. [Google Scholar] [CrossRef]
- Liu, W.G.; Hussain, S.; Liu, T.; Zou, J.L.; Ren, M.L.; Zhou, T.; Liu, J.; Yang, F.; Yang, W.Y. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agric. 2018, 17, 60345–60347. [Google Scholar] [CrossRef]
- Wen, B.X.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.W.; Yang, J.Y.; Xu, M.; Qin, S.S.; Yang, W.Y.; Liu, W.G. Slight shading stress at seedling stage does not reduce lignin biosynthesis or affect lodging resistance of soybean stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef]
- Cashmore, A.R. Cryptochromes: Enabling plants and animals to determine circadian time. Cell 2003, 114, 537–543. [Google Scholar]
- Lin, C.T.; Shalitin, D. Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 2003, 54, 469–496. [Google Scholar] [CrossRef]
- Su, L.; Hou, P.; Song, M.F.; Zheng, X.; Guo, L.; Xiao, Y.; Yan, L.; Li, W.C.; Yang, J.P. Synergistic and antagonistic action of Phytochrome (Phy) A and PhyB during seedling de-Etiolation in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 12199–12212. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Chu, L.; Zhang, Y.M.; Bian, Y.T.; Xiao, J.H.; Xu, D.Q. HY5: A pivotal regulator of light-dependent development in higher plants. Front. Plant Sci. 2021, 12, 800989. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, D.; Gallego-Bartolome, J.; Orlando, L.; Garcia-Carcel, L.; Rubio, V.; Martinez, C.; Frigerio, M.; Iglesias-Pedraz, J.M.; Espinosa, A.; Deng, X.W.; et al. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Smith, D.L.; Liu, W.G.; Chen, X.F.; Yang, W.Y. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr. J. Biotechnol. 2011, 10, 14392–14398. [Google Scholar]
- Xu, T.F.; Yuan, J.H.; Hiltbrunner, A. PHYTOCHROME INTERACTING FACTORs in the moss Physcomitrella patens regulate light-controlled gene expression. Physiol. Plant. 2020, 169, 467–479. [Google Scholar] [CrossRef]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; Lopez-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef]
- de Lucas, M.; Davie’re, J.M.; Rodriguez-Falco’n, M.; Pontin, M.; Lglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blazquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J.A.D. The five “Classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M. Gibberellin insensitive Dwarf1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.T.; Li, J.J.; Wang, B.; Dai, C.; Wang, J.; Liu, K. Brassica napus DS-3, encoding a DELLA protein, negatively regulates stem elongation through gibberellin signaling pathway. Theor. Appl. Genet. 2017, 130, 727–741. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yu, X.H.; Foo, E.; Symons, G.M.; Lopez, J.; Bendehakkalu, K.T.; Xiang, J.; Weller, J.L.; Liu, X.M.; Reid, J.B.; et al. A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol. 2007, 145, 106–118. [Google Scholar] [CrossRef]
- Saniewski, M.; Okubo, H.; Miyamoto, K.; Ueda, J. Auxin induces growth of stem excised from growing shoot of cooled tulip bulbs. J. Fac. Agric. Kyushu Univ. 2005, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.Y.; Kong, S.J.; Chu, X.; Li, X.; Pan, H. De Novo biosynthesis of indole-3-acetic acid in engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 8186–8190. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Law, D.M.; Davies, P.J. Magnitude and kinetics of stem elongation induced by exogenous indole-3-acetic acid in intact light-crown pea seedlings. Plant Physiol. 1993, 102, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Khadr, A.; Wang, G.L.; Li, T.; Wang, Y.H.; Xu, Z.S.; Xiong, A.S. Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci. 2018, 277, 110–120. [Google Scholar] [CrossRef]
- Franck-Duchenne, M.; Wang, Y.W.; Tahar, S.B.; Beachy, R.N. In vitro stem elongation of sweet pepper in media containing 24-epi-brassinolide. Plant Cell Tissue Organ Cult. 1998, 53, 79–84. [Google Scholar] [CrossRef]
- Xie, W.H.; Sun, X.F.; Zhao, L.L.; Huang, L.J.; Wang, P.C. Exogenous hormone application regulates dwarf mutant plant height in Sophora davidii (Franch). Skeels by changing endogenous hormone levels. Chil. J. Agric. Res. 2023, 83, 195–202. [Google Scholar] [CrossRef]
- Yu, X.D.; Cui, X.Y.; Wu, C.; Shi, S.X.; Yan, S.P. Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol. Plant 2022, 11, 1759–1771. [Google Scholar] [CrossRef]
- Kolomiets, M.V.; Hannapel, D.J.; Chen, H.; Tymeson, M.; Gladon, R.J. Lipoxygenase is involved in the control of potato tuber development. Plant Cell 2001, 13, 613–626. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Dijken, A.J.H.V.; Smeekens, S.C.M.; Veldink, G.A.; Vliegenthart, J.F.G. Characterization of three cloned and expressed 13-hydroperoxide lyase isoenzymes from alfalfa with unusual N-terminal sequences and different enzyme kinetics. Eur. J. Biochem. 2000, 267, 2473–2482. [Google Scholar] [CrossRef]
- Li, G.X.; Fu, S.Y.; Chen, Y.; Jin, W.J.; Zhai, B.; Li, Y.F.; Sun, G.R.; Han, R.L.; Wang, Y.B.; Tian, Y.D.; et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int. J. Mol. Sci. 2019, 20, 4063. [Google Scholar] [CrossRef]
- Wang, Z.; Su, G.G.; He, S.L.; Shi, L.Y.; He, D.; Shang, W.Q.; Yang, D.J. Effects of root pruning on adventitious root formation, enzyme activities, and hormone levels in Paeonia sufruticosa ‘Fengdanbai’ Seedlings. Hortic. Sci. Technol. 2021, 39, 10–22. [Google Scholar]
- Hou, J.W.; Guo, S.J.; Wang, G.Y. Effects of in vitro subculture on the physiological characteristics of adventitious root formation in microshoots of Castanea mollissima cv. ‘yanshanhong’. J. For. Res. 2010, 21, 155–160. [Google Scholar] [CrossRef]
- Quan, J.E.; Ni, R.Y.; Wang, Y.; Sun, J.J.; Ma, M.Y.; Bi, H.T. Effects of diferent growth regulators on the rooting of catalpa bignonioides softwood cuttings. Life 2022, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).